- Hong Kong Bat Radar
- Himalayan Leaf-nosed Bat (Hipposideros armiger)
Himalayan Leaf-nosed Bat
Hipposideros armiger (Hodgson, 1835)
Taxonomy
| Family: | Hipposideridae |
| Genus: | Hipposideros |
| Scientific name: | Hipposideros armiger (Hodgson, 1835) |
| Synonyms: | Rhinolophus armiger Hodgson, 1835 |
| Common name: | Himalayan Leaf-nosed Bat |
| Other name: | Great Leaf-nosed Bat, Great/Himalayan Roundleaf Bat |
| Remark: | H. armiger includes four known sub-species: H. a. armiger (Hodgson, 1835), H. a. fujianensis (Zhen, 1987), H. a. terasensis (Kishida, 1924) and H. a. traninhensis (Bourret, 1942). According to geographic distribution, the local species is H. a. armiger. |
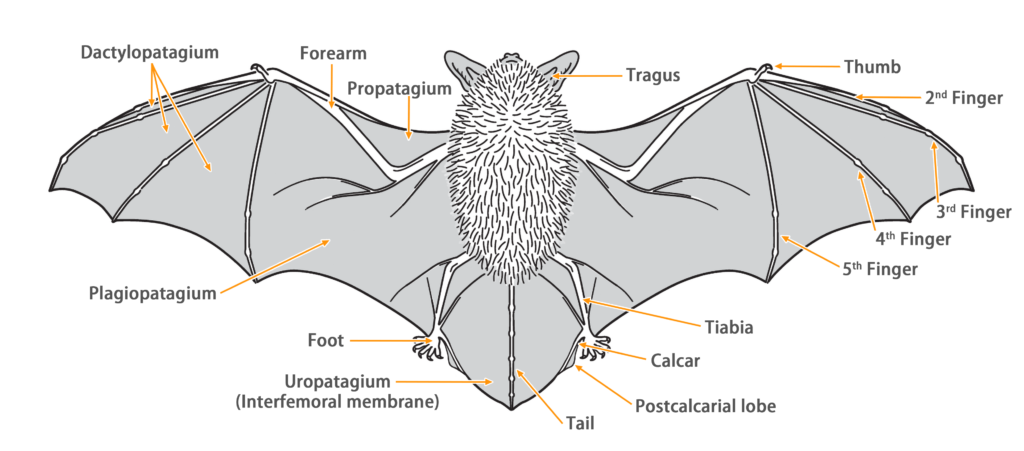
| Characteristics | |
| Color: | There are two color morphs, brown and gray-brown. The dorsal fur color is similar to the ventral fur color. The head fur is relatively darker. Occasionally, individuals with partially whitened fur may be observed. |
| Ear: | The ear pinna is broad, with multiple transverse ridges on the inner side. The ear tips are slightly pointed. Both the ear pinna and antitragus are brown to dark brown in color. The antitragus is short. |
| Head: | The skull is large, and the eyes are small. |
| Noseleaf: | The noseleaf is relatively complex. The anterior leaf is wider and horseshoe-shaped, the intermediate leaf is thick and swollen, and the posterior leaf is thick and shaped like three horizontal lobes. Each side has four supplementary leaflets, outer one being very small. |
| Tail: | The tail is long, completely enveloped by the interfemoral membrane, with a slight protrusion at the tip of the membrane. |
| Other: | There is a transverse opening called the frontal sac behind posterior leaf, with a tuft of black hairs. Both males and females possess this feature, but in females, the frontal sac is mostly covered with fur, while in males, it is more exposed and more developed, appearing milky white. During the breeding season, males may also have black waxy secretions in the frontal sac. |
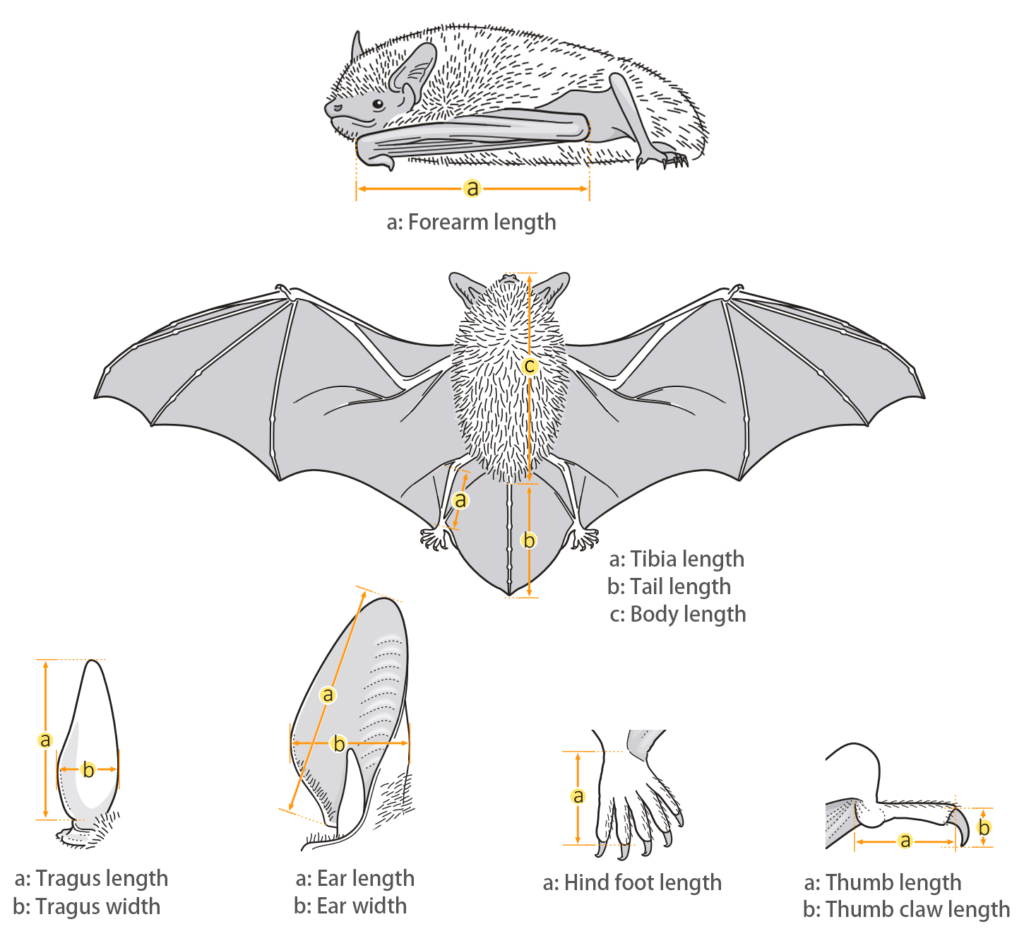
| Body measurements | |
| Size: | Large Leaf-nosed Bat |
| Body: | 80.0 - 110.0 mm |
| Tail: | 48.0 - 70.0 mm |
| Ears: | 30.0 - 35.0 mm |
| Hind foot: | 13.0 - 17.0 mm |
| Forearm: | 85.0 - 103.0 mm |
| Weight: | 44.0 - 67.0 g |
| Wing morphology | |
| Wing span: | 0.532 m |
| Wing area: | 0.050 m2 |
| Wing loading: | 12.21 ± 1.64 N/m2 (High) |
| Aspect ratio: | 5.57 ± 0.31 (Low) |
| Tip-shape index: | 1.58 ± 0.15 (Medium) |
| Reference: | Furey & Racey, 2016 |
Ecology
| Habitat: | This species of cave-dwelling bat can inhabit various types of habitats. During winter, most individuals roost in temperature-stable and spacious water conduits or abandoned mines, while some may choose temperature-stable abandoned buildings for wintering. In summer, some individuals migrate to smaller roosting sites with relatively better air circulation, such as abandoned buildings, shorter water conduits, and tile kilns. They generally prefer locations closer to the entrance/exit of the roost. |
| Habit: | During winter, they form large colonies of tens to thousands of individuals for hibernation, maintaining a certain distance (10-20 cm) between each other. In spring and summer, some individuals leave their original hibernation colonies and migrate to other roosting sites. Summer colonies are typically divided into two categories: "breeding colonies" and "non-breeding colonies." Breeding colonies consist mainly of females and their offspring, with occasional presence of scattered males. Non-breeding colonies are primarily composed of males, although sometimes a few females may be present for maternal care. |
| Reproduction: |
The breeding season occurs from May to June, and females typically give birth to one offspring. Juveniles reach approximately the same size and weight as adult females and become capable of independent flight around 7 weeks after birth. Male bats take one year to reach sexual maturity, while females require two years. The mating period takes place from July to September. Male bats prefer to roost in warmer habitats to enhance their sperm production and prepare for mating, while females ovulate and conceive from July to August. The mating system of this species is seasonal, but it is not yet determined whether it follows a polygynous (one male mates with multiple females) or a promiscuous (multiple males and females mate) mating system. A few more competitive males, such as those with larger body size, join the breeding colonies to increase their mating opportunities and engage in mating with multiple females. |
| Hibernation: | Hibernation typically occurs from late December to February, although the actual duration varies depending on the temperature conditions. |
| Posture: | The typical posture involves extending both wings to support the body while spreading the hind limbs as much as possible, resembling a spread-eagle position against a rock surface. At times, they may also hang vertically with their feet together. |
| Vocalizations: | When startled or disturbed, they emit audible sounds that can be detected by human ears. |
| Flight: | The species exhibits fast flight speed and moderate agility, with good hovering capabilities, although flight efficiency is relatively low, making it more suitable for medium to short-distance flights. They are often observed flying above open canopies or flat terrain and along the edges of their habitat or along corridors such as hiking trails, forest roads, and water conduits. They occasionally rest on branches during flight. |
| Foraging: | This species is nocturnal, with individuals typically leaving the roost about 15 minutes after sunset, foraging throughout the night, and returning before dawn. They continue foraging even in light rain, but remain in the roost during heavy rainfall. |
| Diet: | They have a carnivorous diet, employing various hunting methods including aerial hunting, gleaning, and flycatching. They prefer to prey on medium-sized beetles (15-45mm) such as Phyllophaga, Anomala, Melolontha, and Cyphochilus apicalis. They also consume larger insects from other orders such as Lepidoptera and Hemiptera, including species like Gaeana maculata and Cryptotympana mandarina, and they feed on termites and flying ants. In addition to insects, there have been reports of the presence of horseshoe bat tissues in the feces of this species in Taiwan. Observations in Hong Kong have also documented individuals of this species chewing on feathers of the Dusky Warbler (Phylloscopus plumbeitarsus ). |
Diet composition of H. armiger in China (Guiyang) (Han & He, 2012)
Diet composition of H. armiger in Hong Kong (Shek Kong) (Ades, 1994)
Diet composition of H. armiger in Hong Kong (The Peak) (Ades, 1994)
Life Cycle
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult (Male) | Hibernation | Migrate to summer roosts | Mating |
Migrate to winter roosts and preparing for hibernation |
||||||||
| Adult (Female) | Migrate to maternity roosts | Parturition and maternal care | ||||||||||
|
Caution: |
The above table provides a general overview of the life cycle of the local population. The actual life cycle of each colony can be influenced by factors such as weather conditions, food availability, habitat conditions, and human disturbances. |
Distribution
| Local: | New Territory, Hong Kong Island and Lantau Island |
| Global: | |
| H. a. armiger | Sumatra |
| H. a. fujianensis | Northwest Afghanistan (Nangarhar Province), Pakistan (Khyber Pakhtunkhwa, Punjab, and Sind), India, West & South Sri Lanka, Nepal, Bhutan, Bangladesh, and North Myanmar |
| H. a. terasensis | Borneo (including LabuanI), the Philippines, Sulawesi, SeramI, Ambon Island, and Timor Island |
| H. a. traninhensis | Serasan Island in the Natuna Islands (Monadjem et al., 2019) |
Local distribution map
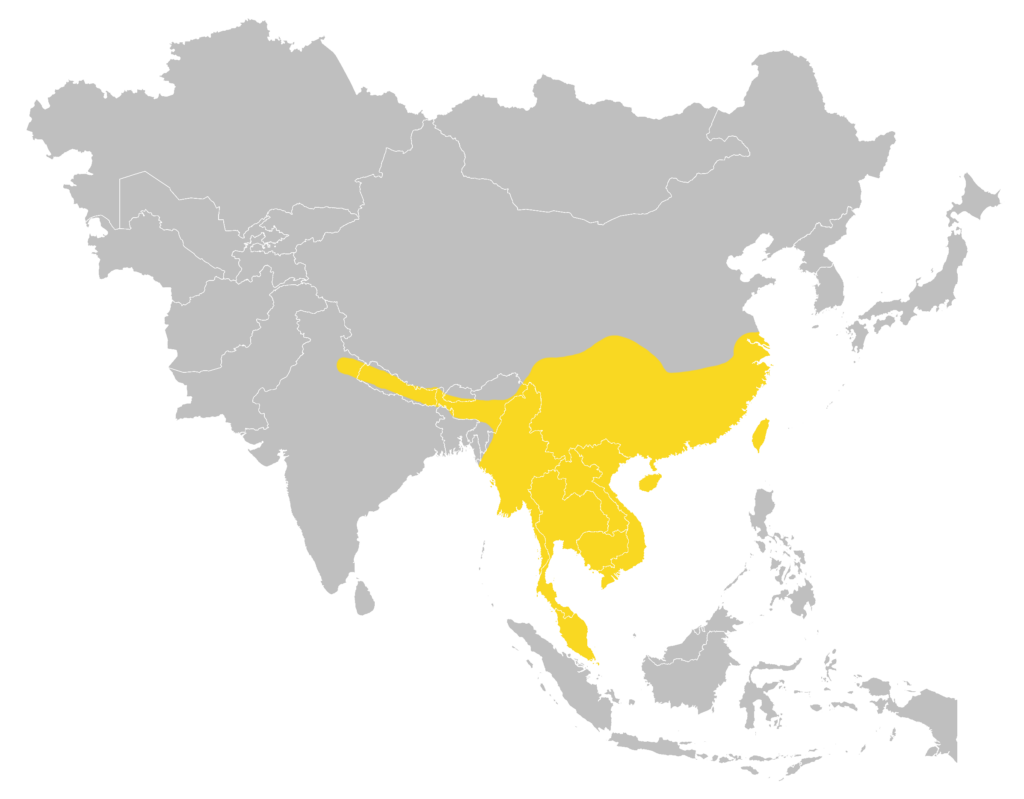
Global distribution map
(Monadjem et al., 2019)
Status and Conservation
| First record: | 1956 |
| Origin: | Native |
| Local status: | Very common (Shek & Chan, 2005) |
| National status: | Least Concern (Red List of China Vertebrates) |
| Global status: | Least Concern (IUCN Red List) |
| Potential threat: | TBC |
Echolocation
| Parameter | Value |
|---|---|
| Call structure | CF-FM |
| Duration | 11.90 ms |
| Inter pulse interval | 44.30 ms |
| Peak frequency | - |
| Highest frequency | 70.10 kHz |
| Lowest frequency | 56.90 kHz |
|
Region: |
Hong Kong |
| Method: | Hand release |
| Reference: | Shek & Lau, 2006 |
| Parameter | Value |
|---|---|
| Call structure | CF-FM |
| Duration | 11.10 ± 2.20 ms |
| Inter pulse interval | 45.70 ± 15.30 ms |
| Peak frequency | 65.00 ± 1.10 kHz |
| Initial frequency | 64.60 ± 1.20 kHz |
| End frequency | 55.70 ± 1.80 kHz |
|
Region: |
Vietnam |
| Method: | Hand release & Flight tent |
| Reference: | Furey et al., 2009 |
| Parameter | Value |
|---|---|
| Call structure | CF-FM |
| Duration | 11.20 ± 2.00 ms |
| Inter pulse interval | 32.60 ± 24.50 ms |
| Peak frequency | 68.60 ± 0.70 kHz |
| Start frequency | - |
| End frequency | - |
|
Region: |
China (Guilin) |
| Method: | Flight tent |
| Reference: | Wei et al., 2011 |
Bibliography
Ades, G. W. J. (1994). A comparative ecological study of insectivorous bats (Hipposideridae, Vespertilionidae and Rhinolophidae) in Hong Kong, with special reference to dietary seasonality. [Doctoral dissertation, The University of Hong Kong].
Bu, Y., Wang, M., Zhang, C., Zhang, H., Zhao, L., Zhou, H., Yu, Y., & Niu, H. (2015). Study of roost selection and habits of a bat, Hipposideros armiger in mainland China. Pakistan Journal of Zoology, 47(1), 59-69.
Cheng, H. C., & Lee, L. L. (2004). Temporal Variations in the Size and Composition of Formosan Leaf-nosed Bat (Hipposideros terasensis) Colonies in Central Taiwan. Zoological Studies, 43(4), 787-794.
Chen, C. H. (1998). Reproductive ecology of Taiwan leaf-nosed bat (Hipposideros terasensis) in Chungliao area, Nantou County. [Master’s Thesis. Tunghai University].
Chen, X. F. (1995). Activity patterns and food habits of sympatric formosan leaf-nosed bat (Hipposideros armiger) and formosan horseshoe bat (rhinolophus monoceros) in Yangmingshan area. [Master’s thesis, National Taiwan University].
Furey, N. M., Mackie, I. J., & Racey, P. A. (2009). The role of ultrasonic bat detectors in improving inventory and monitoring surveys in Vietnamese karst bat assemblages. Current Zoology, 55(5), 327 341.
Furey, N. M., & Racey, P. A. (2016). Can wing morphology inform conservation priorities for Southeast Asian cave bats?. Biotropica, 48(4), 545-556.
Han, B.Y., & He, H.Z. (2012). Study on the Food Composition of Great Leaf-nosed Bats (Hipposideros armiger) and Its Impact on the Occurrence of Forest Pests. Journal of Anhui Agricultural Science, 40(26): 12884-12885.
Ho, Y. Y., & Lee, L. L. (2003). Roost selection by Formosan leaf-nosed bats (Hipposideros armiger terasensis). Zoological Science, 20(8), 1017-1024.
Hughes, A.C., Satasook, C., Bates, P.J.J., Soisook, P., Sritongchuay, T., Jones, G., & Bumrungsri, S. (2011). Using Echolocation Calls to Identify Thai Bat Species: Vespertilionidae, Emballonuridae, Nycteridae and Megadermatidae. Acta Chiropterologica, 13(2), 447–455.
Jiang, Z. G., Jiang, J. P., Wang, Y. Z., Zhang, E., Zhang, Y. Y., Li, L. L., Xie, F., Cai, B., Cao, L., Zheng, G. M., Dong, L., Zhang, Z. W., Ding, P., Luo, Z. H., Ding, C. Q., Ma, Z. J., Tang, S. H., Cao, W. X., Li, C. W., Hu, H. J., Ma, Y., Wu, Y., Wang, Y. X., Zhou, K. Y., Liu, S. Y., Chen, Y. Y., Li, J. T., Feng, Z. J., Wang, Y., Wang, B., Li, C., Song, X. L., Cai, L., Zang, C. X., Zeng, Y., Meng, Z. B., Fang, H. X., & Ping, X. G. (2016). Red List of China’s Vertebrates. Biodiversity Science 24(5), 500‑551.
Monadjem, A., Soisook, P., Thong, V. D., & Kingston, T. (2019). Hipposideridae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats (pp. 227-258). Lynx Edicions.
Shek, C. T. (2006). A Field Guide to the Terrestrial Mammals of Hong Kong. Friends of country park and cosmos book limited.
Shek, C. T., & Chan, C. S. M. (2005). Roost Censuses of Cave Dwelling Bats of Hong Kong. Hong Kong Biodiversity, 10, 1-8.
Stanton, D. J. (2017). Emergence times and seasonality of the great leaf-nosed bat (Hipposideros armiger, Chiroptera: Hipposideridae) in Hong Kong, China. Acta Theriologica Sinica, 37(3), 251.
Tong, C. P. (2016). Distribution and preference of landscape features and foraging sites of insectivorous bats in Hong Kong urban parks. [Master’s dissertation, The University of Hong Kong].
Tsui, W.C., & Chan, W. H. (2023). A Short Note on an Interesting Sighting of Himalayan Leaf-nosed Bat (Hipposideros armiger). Hong Kong Biodiversity, 27, 35-36.
Wei, L., Gan, Y. M., Li, Z. Q., Lin, Z. H., Hong, T. Y., & Zhang, L. B. (2011). Comparisons of echolocation calls and wing morphology among six sympatric bat species. Acta Theriologica Sinica, 31(2), 155-163.
Yang, Y. J. (2011). Mating system and kinship of the formasan leaf-nosed bat. Hipposideros armiger Terasensis (Chiroptera, Hippsideridae). [Marster’s dissertation, National Chung Hsing University].
Zhang, L., Jones, G., Zhang, J., Zhu, G., Parsons, S., Rossiter, S. J., & Zhang, S. (2009). Recent surveys of bats (Mammalia: Chiroptera) from China. I. Rhinolophidae and Hipposideridae. Acta Chiropterologica, 11(1), 71-88.