- Hong Kong Bat Radar
- Least Pipistrelle (Pipistrellus tenuis)
Least Pipistrelle
Pipistrellus tenuis (Temminck, 1840)
Taxonomy
| Family: | Vespertilionidae |
| Genus: | Pipistrellus |
| Scientific name: | Pipistrellus tenuis (Temminck, 1840) |
| Synonyms: |
Pipistrellus portensis J. Allen, 1906, Pipistrellus principulus Thomas, 1915, Vespertilio tenuis Temminck, 1840 |
| Common name: | Least Pipistrelle |
| Other name: | Indian Pygmy Bat |
| Remark: | P. tenuis includes six known sub-species: P. t. tenuis (Temminck, 1840), P. t. mimus (Wroughton, 1899), P. t. nitidus (Tomes, 1859), P. t. portensis (J. A. Allen, 1906), P. t. sewelanus (Oey, 1960) and P. t. subulidens (G. S. Miller, 1901). According to geographic distribution, the local species is P. t. portensis. |
| Characteristics | |
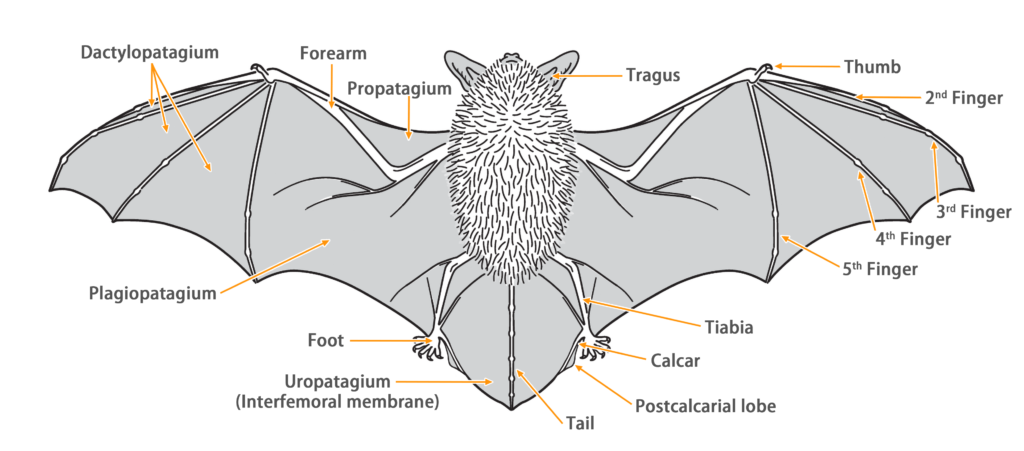
| Color: | The dorsal fur varies, ranging from reddish-brown, dark brown, to blackish-gray, but it is commonly observed as a uniform reddish-brown color (with the base being reddish-brown and the tips being a slightly lighter shade). The forehead region is darker in color. The ventral fur is relatively lighter (with the base being blackish-brown and the tips slightly lighter). Juvenile bats have overall darker fur, appearing gray-black. |
| Ear: | The ear pinna is triangular in shape, with rounded tips. Both the ear pinna and ear tragus are dark brown in color. The ear tragus is thumb-shaped, short and narrow, with a rounded and slightly forward-curved tip, measuring less than half the length of the ear pinna. |
| Head: | The muzzle is wider and appears dark brown to gray-black in color, with prominent lateral swellings that contain glands. The facial and periocular skin generally appears flesh-colored. |
| Limbs: | The hind foot are relatively small, with the wing membrane connecting to the base of the toes. |
| Penis: | The penis is relatively short (approximately 3.82 mm), and the tip of the baculum is forked. |
| Tail: | The tail is long and completely enveloped by the interfemoral membrane, with a slight protrusion (< 1 mm) at the tip beyond the membrane. The calcar is long, extending from the ankle to about half the length of the edge of the uropatagium, with a well-developed postcalcarial lobe. The postcalcarial lobe has a conspicuous transverse cartilaginous septum in the center. |
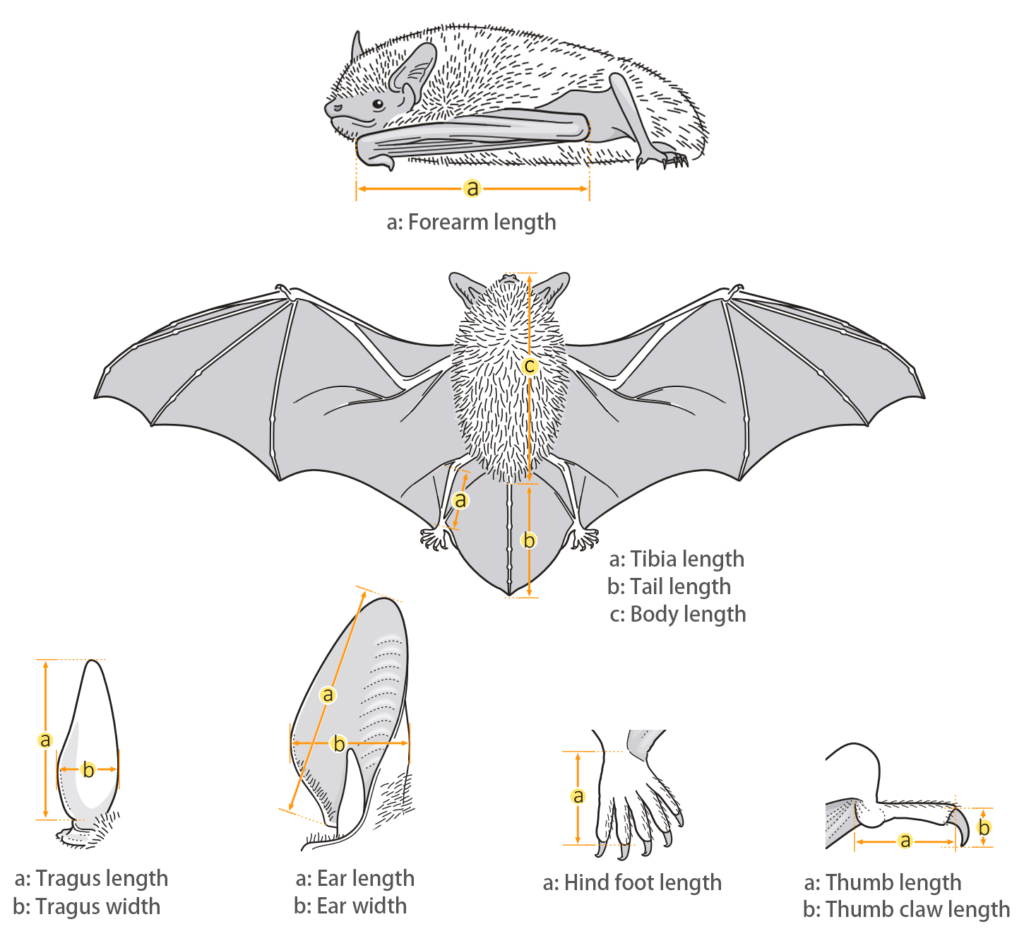
| Body measurements | |
| Size: | Small pipistrelle |
| Body: | 33.0 - 45.0 mm |
| Tail: | 20.0 - 35.0 mm |
| Ears: | 5.0 - 11.0 mm |
| Hind foot: | 3.0 - 7.0 mm |
| Forearm: | 25.0 - 31.0 mm |
| Weight: | 2.9 - 4.2 g |
| Wing morphology | |
| Wing span: | 0.202 m |
| Wing area: | 0.005 m2 |
| Wing loading: | 6.62 ± 1.18 N/m2 (Low) |
| Aspect ratio: | 8.06 ± 0.71 (High) |
| Tip-shape index: | 1.24 ± 0.36 (Low) |
| Refernce: | Jeyapraba et al., 2023 |
Ecology
| Habitat: | They exhibit strong adaptability and can inhabit various habitats, including forests, rural areas, and urban environments. They can roost in tree hollows, hollow branches, and dense foliage, as well as in crevices of tunnels, buildings (rooftops, eaves, wall holes and gaps, information boards, pavilions, etc.), and bat boxes. |
| Habit: | They are known to be gregarious, with colonies typically consisting of 1-25 individuals. Female bats often cohabit with juveniles year-round, while male bats generally maintain solitary roosts. |
| Reproduction: | Pregnant females can be found throughout the year in the Central Indian region, while tropical regions may have two peak breeding seasons. The actual timing of reproduction varies according to the geographical location. Local populations have recorded breeding activity in May and June, with the presence of juvenile bats. |
| Hibernation: | They do not hibernate but exhibit variations in activity levels based on weather temperatures and food availability. |
| Flight: | They have a relatively slow flying speed and limited hovering ability, but possess agility and flight efficiency suitable for medium to short-distance flights. |
| Foraging: | They typically fly at low altitudes (3-5 meters above the ground), often hovering and foraging over tree canopies or bodies of water, as well as maneuvering through forested areas. Occasionally, they may fly as low as 1 meter above the ground while foraging. |
| Diet: | These bats are insectivorous, feeding on insects while in flight. They are considered opportunistic feeders and euryphagous species, adjusting their foraging strategies based on seasonal changes in food availability. In the northwestern region of India, during winter, they primarily feed on beetles, cockroaches, and wingless ants. In the summer months (from March to June), their diet diversifies and includes grasshoppers, crickets, termites, beetles, and moths. During the pre-monsoon season, they predominantly consume termites, moths, hemipterans, and orthopterans. In autumn, their diet shifts to beetles, winged ants, wasps, crickets, grasshoppers, flies, and mosquitoes, among others. |
Diet composition of P. tenuis in South India
(Whitaker et al., 1999)
Diet composition of P. tenuis in Central Nepal (Poudel, 2023)
Distribution
| Local: | New Territory, Hong Kong Island and Lantau Island |
| Global: | |
| P. t. tenuis | Sumatra |
| P. t. mimus | Northwest Afghanistan (Nangarhar Province), Pakistan (Khyber Pakhtunkhwa, Punjab, and Sind), India, West & South Sri Lanka, Nepal, Bhutan, Bangladesh, and North Myanmar |
| P. t. nitidus | Borneo (including LabuanI), the Philippines, Sulawesi, SeramI, Ambon Island, and Timor Island |
| P. t. portensis | South China (including Hainan Island), Myanmar, Thailand, Laos, Vietnam, Cambodia, and Peninsular Malaysia (including Penang Island) |
| P. t. sewelanus | Java, Bali, and Lombok Islands |
| P. t. subulidens | Serasan Island in the Natuna Islands (Moratelli et al., 2019) |
Local distribution map
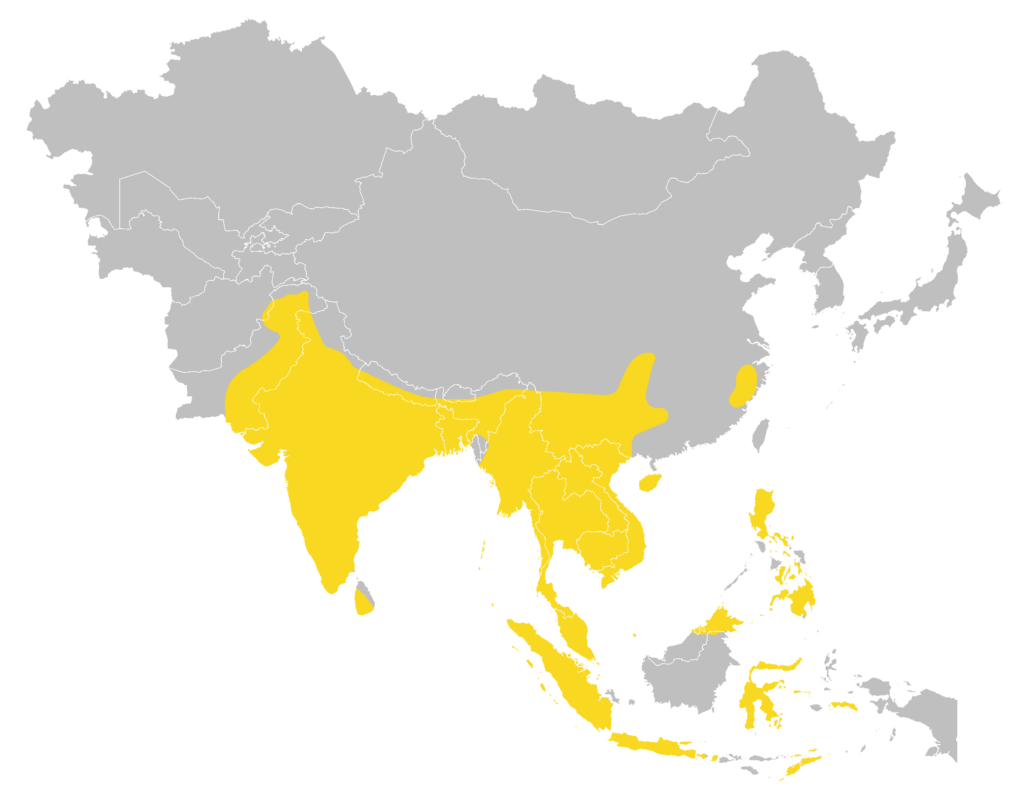
Global distribution map
(Moratelli et al., 2019)
Status and Conservation
| First record: | 2005 |
| Origin: | Native |
| Local status: | Uncommon (Shek & Chan, 2005) |
| National status: | Near Threatened (Red List of China Vertebrates) |
| Global status: | Least Concern (IUCN Red List) |
| Potential threat: | TBC |
Echolocation
| Parameter | Value |
|---|---|
| Call structure | FM/QCF |
| Duration | 3.30 ± 1.10 ms |
| Inter pulse interval | 63.70 ± 38.6 ms |
| Peak frequency | 56.30 ± 6.60 kHz |
| Highest frequency | 87.70 ± 9.80 kHz |
| Lowest frequency | 47.40 ± 4.80 kHz |
| Subspecies: | P. t. minus |
|
Region: |
India |
| Method: | Hand release |
| Reference: | Raghuram et al., 2014 |
| Parameter | Value |
|---|---|
| Call structure | FM/QCF |
| Duration | 5.97 ± 1.53 ms |
| Inter pulse interval | 82.68 ± 9.49 ms |
| Peak frequency | - |
| Highest frequency | 53.30 ± 5.30 kHz |
| Lowest frequency | 46.74 ± 1.94 kHz |
| Subspecies: | N/A |
|
Region: |
N/A |
| Method: | N/A |
| Reference: | Collen, 2012 |
| Parameter | Value |
|---|---|
| Call structure | FM/QCF |
| Duration | - ms |
| Inter pulse interval | - ms |
| Peak frequency | - kHz |
| Highest frequency | - kHz |
| Lowest frequency | - kHz |
| Subspecies: | P. t. portensis |
|
Region: |
Hong Kong |
| Method: | Hand release |
| Reference: | tbc |
Similar Species
Least Pipistrelle
Pipistrellus tenuis
Size:
The smallest among three.
Forearm:
25.0 - 31.0 mm
Color:
Short fur, with a uniformly brownish-black color on the back (slightly lighter at the tips).
Ears:
Triangular in shape, with a dark brown color.
Tragus:
Relatively slender, with a rounded and slightly forward-curved tip.
Snout:
Dark brown to grayish-black, with prominent lateral swellings on both sides.
Tail:
A slight protrusion (< 1 mm) at the tip.
Penis:
Relatively short in length (3.8 mm).
Japanese Pipistrelle
Pipistrellus abramus
Size:
Smaller than P. tenuis and similar to or smaller than H. pulveratus.
Forearm:
29.0 - 36.0 mm
Color:
Short fur, with a gray-brown color on the back (lighter gray-brown at the tips and darker brown at the base).
Ears:
Triangular in shape, with varying degrees of brown coloration.
Tragus:
Relatively slender, with a rounded and slightly forward-curved tip.
Snout:
Dark brown to grayish-black, with slight lateral swellings on both sides.
Tail:
A slight protrusion (< 1 mm) at the tip.
Penis:
A long and distinct penis (9.1 - 12.7 mm).
Chinese Pipistrelle
Hypsugo pulveratus
Size:
Larger than P. tenuis and similar to or larger than P. abramus.
Forearm:
32.0 - 37.0 mm
Color:
Long fur, with a dark brown-black color on the back (slightly lighter at the tips).
Ears:
Slender and triangular in shape, ranging from deep gray to black in color.
Tragus:
Relatively thick and short, with a wider base and a rounded and slightly forward-curved tip.
Snout:
Dark gray to black in color, with slight lateral swellings on both sides.
Tail:
The tail is free for last two vertebrae (~ 3 mm)
Penis:
The shortest penis (2.6 - 3.1 mm) among three.
Bibliography
Collen, A. L. (2012). The evolution of echolocation in bats: a comparative approach [Doctoral dissertation, University of London].
Hughes, A. C., Satasook, C., Bates, P. J. J., Soisook, P., Sritongchuay, T., Jones, G., & Bumrungsri, S. (2011). Using Echolocation Calls to Identify Thai Bat Species: Vespertilionidae, Emballonuridae, Nycteridae and Megadermatidae. Acta Chiropterologica, 13(2), 447–455.
Isaac, S. S., & Marimuthu, G. (1993). Early outflying and late homeflying in the Indian pygmy bat under natural conditions. Oecologia, 96, 426-430.
Jeyapraba, L., Margaret, I. V., Addline, D., & Sakthi, V. (2023). Prediction of foraging strategy of insectivorous bats through their wing morphology. Journal of Survey in Fisheries Sciences, 10(3S), 1,903-1,917.
Jayaraj, V. K., Tahir, N. A., Udin, N. A., Baharin, N. K., Ismail, S. K., & Zakaria, S. N. A. (2012). Species diversity of small mammals at Gunung Stong state park, Kelantan, Malaysia. Journal of Threatened Taxa, 4(6), 2617-2628.
Jiang, Z. G., Jiang, J. P., Wang, Y. Z., Zhang, E., Zhang, Y. Y., Li, L. L., Xie, F., Cai, B., Cao, L., Zheng, G. M., Dong, L., Zhang, Z. W., Ding, P., Luo, Z. H., Ding, C. Q., Ma, Z. J., Tang, S. H., Cao, W. X., Li, C. W., Hu, H. J., Ma, Y., Wu, Y., Wang, Y. X., Zhou, K. Y., Liu, S. Y., Chen, Y. Y., Li, J. T., Feng, Z. J., Wang, Y., Wang, B., Li, C., Song, X. L., Cai, L., Zang, C. X., Zeng, Y., Meng, Z. B., Fang, H. X., & Ping, X. G. (2016). Red List of China’s Vertebrates. Biodiversity Science 24(5), 500‑551.
Moratelli, R., Burgin, C., Cláudio, V., Novaes, R., López-Baucells, A., & Haslauer, R. (2019). Vespertilionidae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats. (pp. 716-981). Lynx Edicions.
Neuweiler, G., Singh, S., & Sripathi, K. (1984). Audiograms of a South Indian bat community. Journal of Comparative Physiology A, 154, 133-142.
Poudel, D. (2023). Species Diversity and Diet Analysis of Insectivorous Bat in Daunne Hill Range, Central Nepal [Doctoral dissertation, Department of Zoology].
Raghuram, H., Jain, M., & Balakrishnan, R. (2014). Species and acoustic diversity of bats in a palaeotropical wet evergreen forest in southern India. Current Science , 107(4), 631-641.
Shek, C. T. (2006). A Field Guide to the Terrestrial Mammals of Hong Kong. Friends of country park and cosmos book limited.
Shek, C. T., & Chan, C. S. M. (2006). Mist Net Survey of Bats with Three New Bat Species Records for Hong Kong. Hong Kong Biodiversity, 11, 1-7.
Tong, C. P. (2016). Distribution and preference of landscape features and foraging sites of insectivorous bats in Hong Kong urban parks. [Master’s thesis, University of Hong].
Thong, V. D., Denzinger, A., Sang, N. V., Huyen, N. T. T., Thanh, H. T., Loi, D. N., Nha, P. V., Viet, N. V., Tien, P. D., Tuanmu, M.-N., Huang, J. C.-C., Thongphachanh, L., Luong, N. T., & Schnitzler, H. U. (2021). Bat Diversity in Cat Ba Biosphere Reserve, Northeastern Vietnam, A Review with New Records from Mangrove Ecosystem. Diversity, 13(8), 376.
Whitaker Jr, J. O., Issac, S. S., Marimuthu, G., & Kunz, T. H. (1999). Seasonal variation in the diet of the Indian pygmy bat, Pipistrellus mimus, in southern India. Journal of Mammalogy, 80(1), 60-70.
Zhang, J. S. (2010). The bats (Mammalia: Chiroptera) of China: an integrative approach to the taxonomy [Doctoral dissertation, Graduate University of the Chinese Academy of Sciences].
Zhou, J., Yang, T. Y., & Hou, X. F. (2011). The Least Pipistrelle (Pipistrellus tenuis) was Discovered in Guizhou Province. Chinese Journal of Zoology, 46(1), 115-119.
Hong Kong Bat Radar. (01/05/2024). A Field Guide to Bats of Hong Kong: Least Pipistrelle (Pipistrellus tenuis ). https://hkbatradar.com/en/pipistrellus_tenuis