- Hong Kong Bat Radar
- Whiskered Myotis (Myotis muricola)
Whiskered Myotis
Myotis muricola (Gray, 1846)
Taxonomy
| Family: | Vespertilionidae |
| Genus: | Myotis |
| Scientific name: | Myotis muricola (Gray, 1846) |
| Synonyms: |
Myotis mystacinus ssp. caliginosus (Tomes, 1859) Myotis mystacinus ssp. muricola (Gray, 1846) Vespertilio blanfordi Dobson, 1871 Vespertilio caliginosus Tomes, 1859 Vespertilio muricola Gray, 1846 Vespertilio muricola Hodgson, 1841 [nomen nudum] |
| Common name: | Whiskered Myotis |
| Other name: | Dark Whiskered Bat, Nepalese Whiskered Bat |
| Remark: |
M. muricola was initially considered a subspecies of M. mystacinus and was officially recognized as a separate species in 1846. At that time, there were up to eight known subspecies (browni, caliginosus, herrei, latirostris, moupinesis, muricola, niasensis, and patriciae) (Simmons, 2005). Although M. muricola remains a complex taxon that requires further investigation, advancements in molecular genetics have shed light on its classification. Recent studies have suggested that moupinesis, caliginosus, and latirostris should be classified under the genus Submyotodon, specifically as S. moupinesis, S. caliginosus, and S. latirostris (Ruedi et al., 2015). Furthermore, the browni, herrei, and patriciae populations in the Philippines are now considered a separate and distinct species, M. browni (Don & Russell, 2019). Furthermore, another study by Wiantoro et al. (2012) revealed that the average genetic distance between M. muricola populations on either side of Wallace's Line reaches up to 31.5%, suggesting the likely presence of two cryptic species. Additionally, there is an average genetic distance of 7.2% among populations west of Wallace's Line, allowing for regional subdivision into two subgroups: Sumatra-Asia and Borneo. The M. muricola population in Hong Kong belongs to the Sumatra-Asia subgroup. |
| Characteristics | |
| Color: | The fur is dense, with a uniform dark brown or blackish-brown color on the dorsal side (dark brown or black bases, and dark brown tips), interspersed with long hairs that have a gray-white or light tan color at their tips. The ventral fur exhibits a lighter coloration, appearing as gray-white (dark brown to black bases and gray-white tips). Juvenile bats generally have a darker overall fur color, appearing gray-black. |
| Ear: | The ear pinnae are elongated, with the upper portion (approximately 1/4) of the anterior and posterior edges noticeably narrowing. The middle section of the posterior edge has a distinct notch, and the ear tips are rounded. Both the ear pinnae and tragus are deep gray in color. The tragus are slender and conical, broader at the base, and less than half the length of the ear pinnae. |
| Head: | The facial fur is short and sparse, appearing blackish-brown. The skin of the muzzle and face are reddish grey in color, and the snout is darker and adorned with well-developed whiskers. |
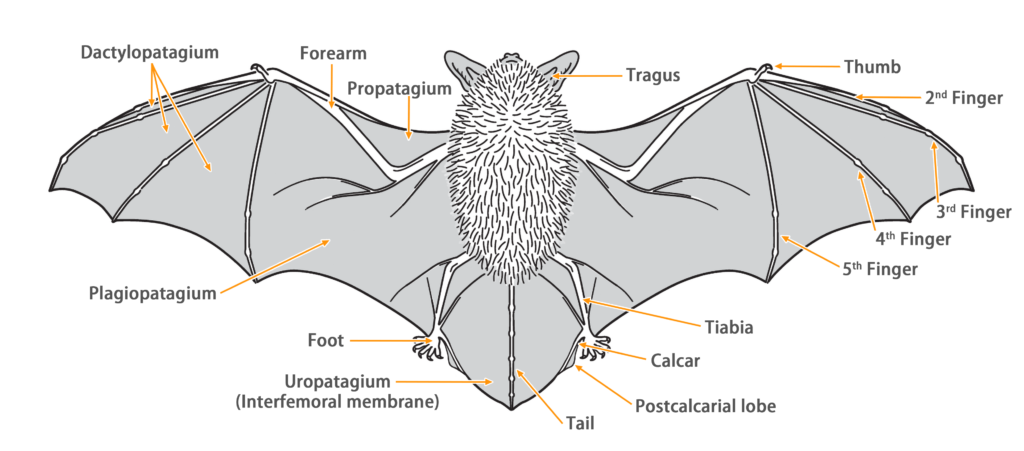
| Limbs: | The hind limbs are slender and shorter than half the length of the tibia. The wing membranes connect to the base of the toes by at least 1mm, while the tail membrane attaches to the ankle. |
| Tail: | The tail is long and completely enveloped by the interfemoral membrane, with a slightly protruding tip (approximately 0.6 mm) beyond the membrane. The calcar is over halfway to tail from ankle and has well-developed postcalcarial lobe. |
| Body measurements | |
| Size: | Small myotis |
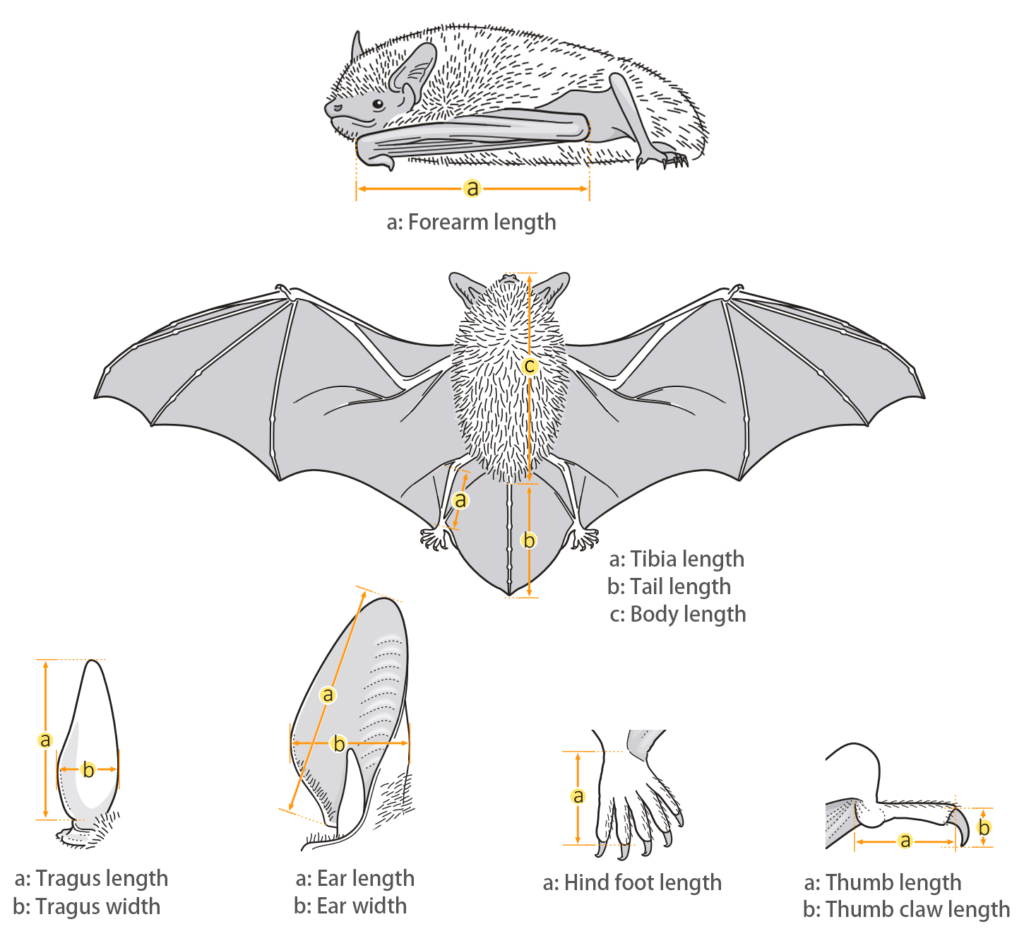
| Body: | 41.0 - 51.0 mm |
| Tail: | 35.0 - 49.0 mm |
| Ears: | 10.6 - 14.6 mm |
| Hind foot: | 4.6 - 8.0 mm |
| Forearm: | 31.2 - 38.6 mm |
| Weight: | 3.2 - 6.5 g |
| Wing morphology | |
| Wing span: | 0.263 m |
| Wing area: | 0.009 m2 |
| Wing loading: | 5.70 N/m2 (Very Low) |
| Aspect ratio: | 7.30 (High) |
| Tip-shape index: | - |
| Reference: | Pottie et al., 2004 |
Ecology
| Habitat: | Cave-dwelling and tree-roosting bats. They typically inhabit the rolled-up leaves at the center of banana trees, and when the leaves unfurl, they move to other rolled leaves or habitats. They can also be found roosting in irrigation channels, abandoned mines, old buildings, and tree cavities. |
| Habit: | Colonies roosting in banana tree leaves are generally small in size (1-8 individuals) and temporary, relocating as the leaves gradually unfold. They prefer choosing higher and unopened rolled leaves for roosting. Colonies roosting in more stable caves or tree cavities tend to be larger in size. |
| Reproduction: | Breeding season occurs from May to June each year, with typically one offspring produced. Local breeding records have been observed in banana tree leaves. |
| Hibernation: | Hibernation usually takes place from late December to February, with the actual duration varying depending on temperature conditions. |
| Migration: | No migration records. |
| Flight: | They have a relatively slow flight speed (low wing beat frequency and long intervals between echolocation calls), but exhibit high flight maneuverability and efficiency, suitable for medium-distance flights. |
| Foraging: | They are accustomed to foraging in dense forests, along forest paths, and near streams, as well as preying on insects around street lights on the outskirts of suburban areas. |
| Diet: | Insectivorous bats, capturing small to medium-sized insects in mid-air. They primarily prey on insects from the orders Lepidoptera and Coleoptera. They can use their wing and tail membranes to assist in capturing larger insects, and can also directly prey on smaller prey such as mosquitoes (Aedes albopictus). |
Diet composition of M. muricola in Thailand (Aroon, 2014)
Distribution
| Local: | New Territory, Hong Kong Island and Lantau Island |
| Global: | Nepal, East & Northeast India (including Sikkim), Bhutan, Myanmar, Southeast China (Guizhou, Hunan, and Guangxi), Southeast Asia, Borneo (including Bunguran Is), Sumatra (including Simeulue, Nias, and Sipora Islands), Java, Bali, Lesser Sundas (Lombok, Sumbawa, Sumba, and Flores Islands), and Central Moluccas (Ambon Island). (Don & Russell, 2019) |
Local distribution map

Global distribution map
(Moratelli et al., 2019)
Status and Conservation
| First record: | 2005 |
| Origin: | Native |
| Local status: | Rare (Shek & Chan, 2005) |
| National status: | Near Threatened (Red List of China Vertebrates) |
| Global status: | Least Concern (IUCN Red List) |
| Potential threat: | TBC |
Echolocation
| Parameter | Value |
|---|---|
| Call structure | FM |
| Duration | 1.90 ms |
| Inter pulse interval | 58.20 ms |
| Peak frequency | 66.20 kHz |
| Start frequency | 97.80 kHz |
| End frequency | 54.50 kHz |
|
Region: |
Vietnam |
| Method: | Hand release |
| Reference: | Furey et al., 2009 |
| Parameter | Value |
|---|---|
| Call structure | FM |
| Duration | 4.98 ± 0.07 ms |
| Inter pulse interval | - |
| Peak frequency | 57.20 ± 0.01 kHz |
| Start frequency | 79.90 ± 1.02 kHz |
| End frequency | 53.70 ± 0.01 kHz |
|
Region: |
Singapore |
| Method: | Flight tent/Hand release |
| Reference: | Pottie et al., 2004 |
| Parameter | Value |
|---|---|
| Call structure | FM-QCF |
| Duration | 4.10 ± 0.20 ms |
| Inter pulse interval | 102.40 ± 27.90 ms |
| Peak frequency | 56.1 ± 2.5 kHz |
| Highest frequency | 90.80 ± 23.10 kHz |
| Lowest frequency | 49.80 ± 1.60 kHz |
|
Region: |
Malaysia |
| Method: | Hand release |
| Reference: | McArther & Khan, 2021 |
Similar Species

Horsfield's Myotis
Myotis horsfieldii
Size:
Similar body sizes.
Color:
The dorsal fur displays a mottled pattern of dark grayish-brown coloration, while the ventral fur ranges from grayish-white to dark gray.
Ear pinna:
The upper portion of the anterior and posterior edges of the ear pinnae slightly narrows.
Tragus:
Slender, elongated, and overall relatively small in size.
Hindfoot:
Relatively large, with a length exceeding half the length of the tibia.
Wing membranes:
Connect to the base of the toes at a distance of approximately 2 mm from the ankle.

Whiskered Myotis
Myotis muricola
Size:
Similar body sizes.
Color:
The dorsal fur displays a uniform dark brown to blackish-brown coloration, while the ventral fur ranges from grayish-white to grayish-brown.
Ear pinna:
The upper portion of the anterior and posterior edges of the ear pinnae noticeably narrows, and there is a distinct notch in the middle section of the posterior edge.
Tragus:
Slender, conical, and overall narrower in shape.
Hindfoot:
Relatively smaller, with a length that is less than half the length of the tibia.
Wing membranes:
Connect to the base of the toes at a distance of more than 1 mm from the ankle.
Bibliography
Aroon, S. (2014). Community, diet, and ectoparasites of bats in Sakaerat Environmental Research station, Nakhon Ratchasima province. [Doctoral dissertation, School of Environmental Biology Institute of Science Suranaree University of Technology].
Baker, N. (2023). Bats of SE Asia – Whiskered Myotis. Ecology Asia. https://www.ecologyasia.com/verts/bats/whiskered-myotis.html
Benda, P. (2010). On a small collection of bats (Chiroptera) from western Sabah (North Borneo, East Malaysia). Vespertilio, 13(14), 45-76.
Francis, C. M., & Hill, J. E. (1998). New records and a new species of Myotis (Chiroptera, Vespertilionidae) from Malaysia. Mammalia, 62(2), 241-252.
Heaney, L. R., Balete, D. S., & Rickart, E. A. (2016). The mammals of Luzon Island: biogeography and natural history of a Philippine fauna. JHU Press: 248-249.
Hughes, A. C., Satasook, C., Bates, P. J. J., Soisook, P., Sritongchuay, T., Jones, G., & Bumrungsri, S. (2011). Using Echolocation Calls to Identify Thai Bat Species: Vespertilionidae, Emballonuridae, Nycteridae and Megadermatidae. Acta Chiropterologica, 13(2), 447–455.
Jennings, N. V., Parsons, S., Barlow, K. E., & Gannon, M. R. (2004). Echolocation calls and wing morphology of bats from the West Indies. Acta Chiropterologica, 6(1), 75-90.
Jiang, Z. G., Jiang, J. P., Wang, Y. Z., Zhang, E., Zhang, Y. Y., Li, L. L., Xie, F., Cai, B., Cao, L., Zheng, G. M., Dong, L., Zhang, Z. W., Ding, P., Luo, Z. H., Ding, C. Q., Ma, Z. J., Tang, S. H., Cao, W. X., Li, C. W., Hu, H. J., Ma, Y., Wu, Y., Wang, Y. X., Zhou, K. Y., Liu, S. Y., Chen, Y. Y., Li, J. T., Feng, Z. J., Wang, Y., Wang, B., Li, C., Song, X. L., Cai, L., Zang, C. X., Zeng, Y., Meng, Z. B., Fang, H. X., & Ping, X. G. (2016). Red List of China’s Vertebrates. Biodiversity Science 24(5), 500‑551.
McArthur, E., & Khan, F. A. A. (2021). Towards a regional call library: classifying calls of a species-rich bat assemblage in a Bornean karst rainforest. Journal of Bat Research & Conservation, 14(1), 95-117.
Moratelli, R., Burgin, C., Cláudio, V., Novaes, R., López-Baucells, A., & Haslauer, R. (2019). Vespertilionidae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats. (pp. 716-981). Lynx Edicions.
Pottie, S. A., Lane, D. J., Kingston, T., & Lee, B. P. H. (2005). The microchiropteran bat fauna of Singapore. Acta Chiropterologica, 7(2), 237-247.
Ruedi, M., Csorba, G., Lin, L. K., & Chou, C. H. (2015). Molecular phylogeny and morphological revision of Myotis bats (Chiroptera: Vespertilionidae) from Taiwan and adjacent China. Zootaxa, 3920(1), 301-342.
Ruedi, M., Saikia, U., Thabah, A., Görföl, T., Thapa, S., & Csorba, G. (2021). Molecular and morphological revision of small Myotinae from the Himalayas shed new light on the poorly known genus Submyotodon (Chiroptera: Vespertilionidae). Mammalian Biology, 101(4), 465-480.
Shek, C. T. (2006). A Field Guide to the Terrestrial Mammals of Hong Kong. Friends of country park and cosmos book limited.
Shek, C. T. & Chan, C. S. M. (2006). Mist Net Survey of Bats with Three New Bat Species Records for Hong Kong. Hong Kong Biodiversity 11, 1-7.
Simmons, N. B. (2005). Order chiroptera. Mammal species of the world: a taxonomic and geographic reference, 1, 312-529.
Wiantoro, S., Maryanto, I. , & Abdullah, M. T. (2012). Phylogeny and phylogeography of Myotis muricola (Gray, 1846 ) (Chiroptera: Vespertilionidae) from the West and East of Wallace’s Line inferred from partial mtDNA cytochrome b gene. Pertanika Journal of Tropical Agricultural Science, 35(2), 271-292.
Hong Kong Bat Radar. (01/05/2024). A Field Guide to Bats of Hong Kong: Whiskered Myotis (Myotis muricola ). https://hkbatradar.com/en/myotis_muricola