- Hong Kong Bat Radar
- Wrinkle-lipped free-tailed bat (Mops plicatus)
Wrinkle-lipped Free-Tailed Bat
Mops plicatus (Buchannan, 1800)
Taxonomy
| Family: | Molossidae |
| Genus: | Mops |
| Scientific name: | Mops plicatus (Buchannan, 1800) |
| Synonyms: |
Chaerephon plicatus (Buchanan, 1800), Dysopes murinus Gray, 1830, Nyctinomus bengalensis Desmarest, 1820, Tadarida plicata ssp. insularis Phillips, 1932, Vespertilio plicatus Buchannan, 1800 |
| Common name: | Wrinkle-lipped Free-tailed Bat |
| Other name: | Wrinkle-lipped Bat |
| Remark: | The genera Chaerephon and Mops, previously classified as separate genera, have recently been recognized as a single genus based on molecular phylogenetic studies of the family Molossidae (Lamb et al., 2011; Ammerman et al., 2012, 2013; Amador et al., 2016). These taxonomic studies have determined that the two genera should be subsumed under the monophyletic genus Mops  Lesson, 1842, which has nomenclatural priority. |
| Characteristics | |
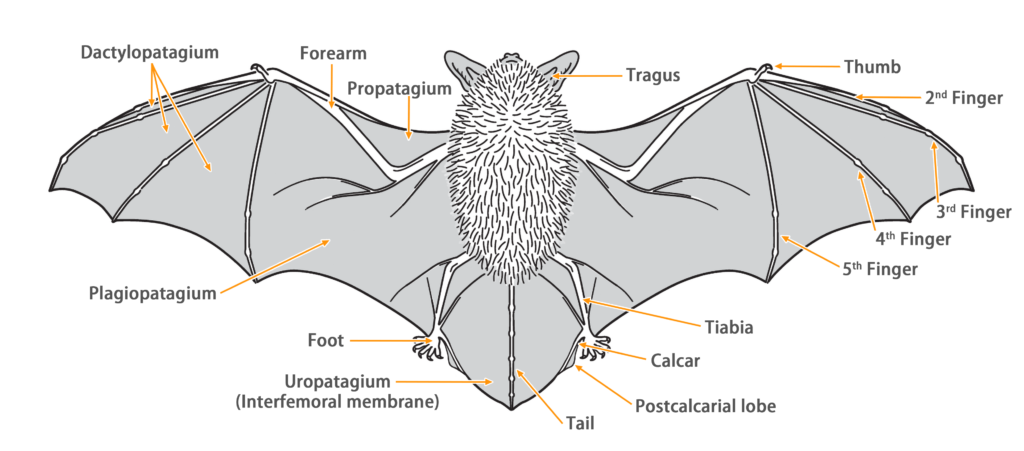
| Color: | The fur is short, dense, and soft. The dorsal fur is uniform dark brown or grayish-black color, while the ventral fur is paler (dark brown bases and grayish or white tips). |
| Ear: | The pinnae are broad and thick, projecting forward, with the anterior borders connected with a skin fold across the forehead. The anterior edge of the pinna forms a slightly flattened triangle. There are six transverse ridges on the posterior lobe. The tip is blunt with several papillae over the ears. The antitragus is well-developed and prominent, but the tragus is very small and completely concealed by the antitragus. Both the pinna and tragus are grayish-black. |
| Head: | Nostrils are slightly protruded and open sidewards, and its upper edge is surrounded by a single row of dermal papillae. The upper lip is well developed with longitudinal wrinkles. The muzzle and wrinkles on the upper lip have a few black thornlike hairs. Their eyes are relatively large. |
| Limbs: | The hind feet are small, with the toes and claws partially covered by long hairs. The first and fifth toes are more robust, with a row of white, spoon-shaped hairs along the outer edge (a dense layer of short, spoon-shaped hairs interspersed with sparse, longer spoon-shaped hairs). The wing membrane is attached to the bottom of the tibia. |
| Tail: | Their tail is approximately half the body length, thick and hairless, with more than half protruding from the narrow and naked uropatagium. The calcar is well-developed and unkeeled, extending from the ankle to two-thirds of the outer edge of the uropatagium. |
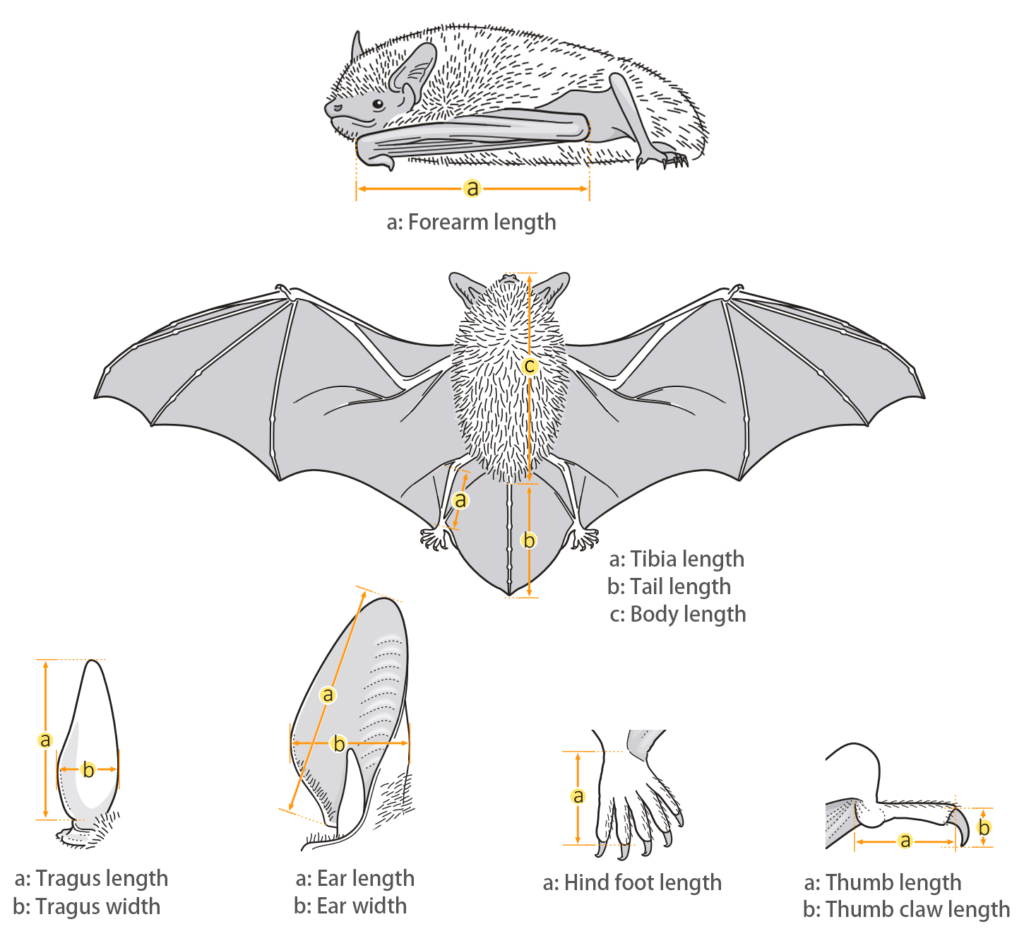
| Body measurements | |
| Size: | Medium Free-tail Bat |
| Body: | 65.0 - 75.0 mm |
| Tail: | 28.0 - 40.0 mm |
| Ears: | 16.0 - 24.0 mm |
| Hind foot: | 8.0 - 12.0 mm |
| Forearm: | 40.0 - 50.0 mm |
| Weight: | 13.0 - 31.0 g |
| Wing morphology | |
| Wing span: | 0.202 m |
| Wing area: | 0.005 m2 |
| Wing loading: | 20.58 - 28.42 N/m2 (High) |
| Aspect ratio: | 15.47 - 19.08 (High) |
| Tip-shape index: | - |
| Reference: | Leelapaibul et al., 2005 |
Ecology
| Habitat: | Cave-dwelling bats, with large colonies generally roosting in karst caves, while relatively smaller colonies inhabit rock crevices, abandoned buildings, and temples. |
| Habit: | They are gregarious, typically forming very large colonies ranging from hundreds to millions of individuals. The largest seasonal colony in Vietnam is found in a cave in Lạng Sơn Province, estimated at over 3 million individuals. The largest colony in Thailand resides in the Khao Chong Pran Cave at Wat Khao Chong Pran, estimated at 2.6 million individuals. The largest colony in Malaysia is in the Gomantong Caves, Sabah, estimated at 270,000 individuals. In China, the largest recorded colony is in the Tianshengqiao Cave, Libo County, Guizhou Province, estimated at 100,000 individuals. Locally, no cave roosts have been recorded, with only scattered individual records, mostly of individuals that have accidentally entered buildings. |
| Reproduction: | They have two annual breeding cycles. In Thailand, Myanmar, and Cambodia, the gestation periods are February-March and August-September, with parturition primarily occurring in April and October, producing one pup per litter. |
| Hibernation: | They are active year-round and do not hibernate. |
| Flight: | They have narrow, elongated wings (with very high wing loading and aspect ratio), enabling them to fly at extremely fast speeds (average 10.38 ± 0.85 m/s) with excellent flight efficiency, suitable for long-distance flight. Although their maneuverability is relatively poor, they possess good agility and can execute sharp turns, climbs, and dives while flying at high speeds. They typically fly at high speeds in a straight line at high altitudes, preferring open areas (farmlands, rice fields). When emerging from the roost, they leave collectively, forming a continuous, elongated band in the air. When returning to the roost, they first congregate approximately 0.5-1 km away, ascend to about 200 m, and then dive back into the cave at high speeds (up to 20 m/s). |
| Foraging: | They are nocturnal, beginning their collective foraging bouts before dusk. Their foraging range is extensive, with a diameter of approximately 27 km. Their foraging height ranges from the ground up to 200 m above the surface. This height varies with time and the stability of the boundary layer: from dusk until midnight, they typically forage between the ground and 100 m; from midnight until dawn, they gradually ascend to forage at 200 m. In the evenings, they generally forage in open areas near the ground, such as rice fields, open water bodies, and above the canopy layer. |
| Diet: | Insectivorous, capturing insects in flight. Their primary prey includes Isoptera, Lepidoptera, Hemiptera, and Coleoptera, with a preference for Isoptera, such as the white-backed planthopper (Sogatella furcifera ) and the brown planthopper (Nilaparvata lugens ). Females tend to prefer Lepidoptera and Coleoptera over males and consume fewer Odonata. |
Diet composition of M. plicatus in central Thailand (Thongjued et al., 2018)
Diet composition of M. plicatus in central Thailand (Leelapaibul et al., 2005)
Diet composition of M. plicatus in central Thailand (Srilopan, 2018)
Distribution
| Local: | Potentially wide distributed in Hong Kong |
| Global: | India, Sri Lanka, Myanmar, China, Thailand, Laos, Vietham, Cambodia, Borneo, Java, Lesser Sunda Islands (Bali and Lombok), and Philippines; presence in Bangladesh is very likely but needs confirmation. Previous records on Malay Peninsula and Sumatra need further verification as to whether the populations may have disappeared or may have been misidentified. (Taylor et al., 2019) |
Local distribution map
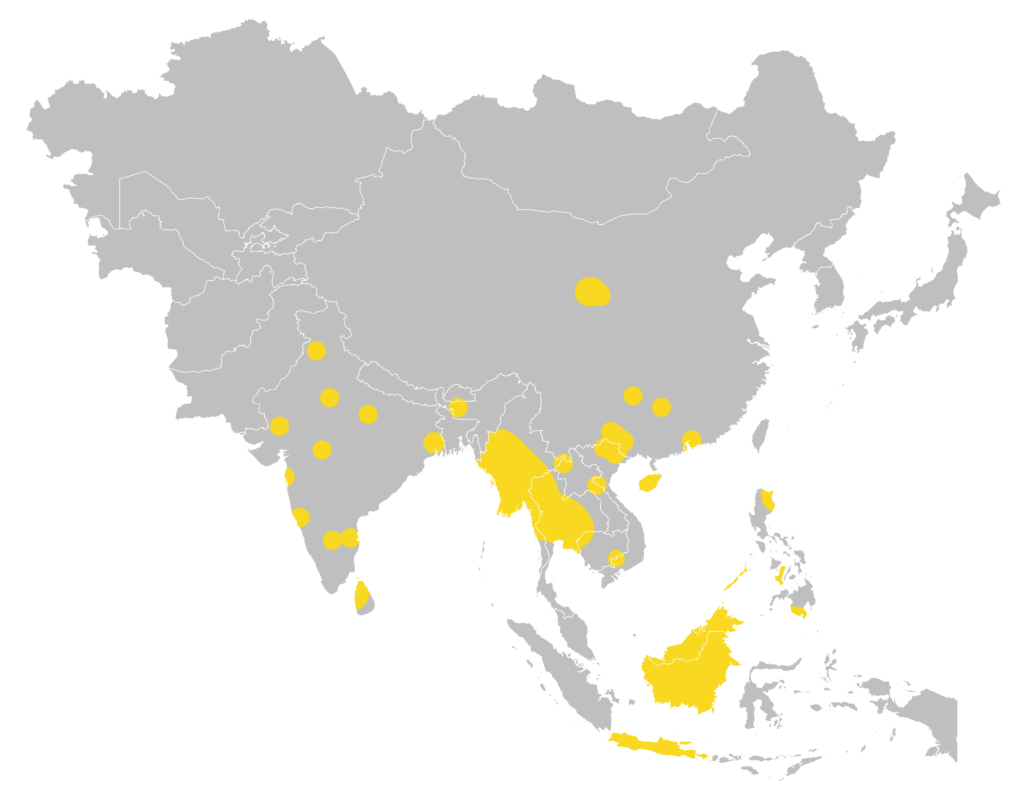
Global distribution map
(Taylor et al., 2019)
Status and Conservation
| First record: | 1957 |
| Origin: | Native |
| Local status: | Rare (Shek, 2006a) |
| National status: | Least Concern (Red List of China Vertebrates) |
| Global status: | Least Concern (IUCN Red List) |
| Potential threat: | TBC |
Echolocation

| Parameter | Value |
|---|---|
| Call structure | QCF-FM |
| Duration | 7.70 ms |
| Inter pulse interval | 85.20 ms |
| Peak frequency | 27.60 kHz |
| Highest frequency | 39.70 kHz |
| Lowest frequency | 17.80 kHz |
|
Region: |
Sri Lanka |
| Method: | Hand release |
| Reference: | Kusuminda & Yapa, 2017 |
| Parameter | Value |
|---|---|
| Call structure | QCF-FM |
| Duration | 7.0 ± 3.4 ms |
| Inter pulse interval | - |
| Peak frequency | - |
| Start frequency | 38.40 ± 2.60 kHz |
| End frequency | 12.60 ± 2.10 kHz |
|
Region: |
Vietnam |
| Method: | Hand release/Wild call |
| Reference: | Thong et al., 2022 |
| Parameter | Value |
|---|---|
| Call structure | FM |
| Duration | 12.31 ms |
| Inter pulse interval | 147.2 ms |
| Peak frequency | 23.65 kHz |
| Start frequency | 35.88 kHz |
| End frequency | 19.76 kHz |
|
Region: |
India |
| Method: | Wild call |
| Reference: | Deshpande & Kelkar, 2015 |
Bibliography
Amador, L.I., Moyers Arévalo R.L., Almeida F.C., Catalano S. A., & Giannini, N.P. (2016). Bat systematics in the light of unconstrained analyses of a comprehensive molecular supermatrix. Journal of Mammalian Evolution 2016, 1-34.
Ammerman, L. K., Brashear, W. A., & Bartlett, S.N. (2013). Further evidence for the basal divergence of Cheiromeles (Chiroptera: Molossidae). Acta Chiropterologica, 15(2), 307-312.
Ammerman, L. K., Lee, D. N., & Tipps, T. M. (2012). First molecular phylogenetic insights into the evolution of free-tailed bats in the subfamily Molossinae (Molossidae, Chiroptera). Journal of Mammalogy, 93(1), 12-28.
Deshpande, K., & Kelkar, N. (2015). Acoustic identification of Otomops wroughtoni and other free-tailed bat species (Chiroptera: Molossidae) from India. Acta Chiropterologica, 17(2), 419-428.
Gregorin, R., & Cirranello, A. (2016). Phylogeny of Molossidae Gervais (Mammalia: Chiroptera) inferred by morphological data. Cladistics, 32(1), 2-35.
Hillman, A. (1999). The study on wrinkled-lipped free-tailed bats (Tadarida plicata) at Khao Chong Pran Non-hunting Area, Ratchaburi Province. Royal Forest Department Journal, 1(1), 72-83.
JOHNSON, C. G. 1969. Migration and dispersal
Lamb, J. M., Ralph, T., Naidoo, T., Taylor, P. J., Ratrimomanarivo, F., Stanley, W. T., & Goodman, S. M. (2011). Toward a molecular phylogeny for the Molossidae (Chiroptera) of the Afro-Malagasy region. Acta Chiropterologica, 13(1): 1-16.
Leelapaibul, W., Bumrungsri, S., & Pattanawiboon, A. (2005). Diet of wrinkle-lipped free-tailed bat (Tadarida plicata Buchannan, 1800) in central Thailand: insectivorous bats potentially act as biological pest control agents. Acta Chiropterologica, 7(1), 111-119.
Jeyapraba, L., Margaret, I. V., Addline, D., & Sakthi, V. (2023). Prediction of foraging strategy of insectivorous bats through their wing morphology. Journal of Survey in Fisheries Sciences, 10(3S), 1,903-1,917.
Jiang, Z. G., Jiang, J. P., Wang, Y. Z., Zhang, E., Zhang, Y. Y., Li, L. L., Xie, F., Cai, B., Cao, L., Zheng, G. M., Dong, L., Zhang, Z. W., Ding, P., Luo, Z. H., Ding, C. Q., Ma, Z. J., Tang, S. H., Cao, W. X., Li, C. W., Hu, H. J., Ma, Y., Wu, Y., Wang, Y. X., Zhou, K. Y., Liu, S. Y., Chen, Y. Y., Li, J. T., Feng, Z. J., Wang, Y., Wang, B., Li, C., Song, X. L., Cai, L., Zang, C. X., Zeng, Y., Meng, Z. B., Fang, H. X., & Ping, X. G. (2016). Red List of China’s Vertebrates. Biodiversity Science 24(5), 500‑551.
Kusuminda, T., & Yapa, W. B. (2017). First record of a Wrinkle-lipped Free-tailed Bat Chaerephon plicatus Buchannan, 1800 (Mammalia: Chiroptera: Molossidae) colony in Sri Lanka, with notes on echolocation calls and taxonomy. Journal of Threatened Taxa, 9(4), 10115-10120.
Ngoc Nguyen, T. (2018). Vertical stratification in foraging activity of the wrinkle-lipped free-tailed bat, chaerephon plicatus (buchanan, 1800) in Central Thailand. [Doctoral dissertation, Prince of Songkla University].
McFarlane, D. A., Van Rentergem, G., Ruina, A., Lundberg, J., & Christenson, K. (2015). Estimating colony size of the wrinkle-lipped bat, Chaerephon plicatus (Chiroptera: Molossidae) at Gomantong, Sabah, by quantitative image analysis. Acta Chiropterologica, 17(1), 171-177.
Norberg, U. M., & Rayner, J. M. (1987). Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 316(1179), 335-427.
Shek, C. T. (2006)a. A Field Guide to the Terrestrial Mammals of Hong Kong. Friends of country park and cosmos book limited.
Shek, C. T. (2006)b. Wrinkle-lipped Free-tailed Bat (Chaerephon plicata 皺唇犬吻蝠) in Hong Kong . Hong Kong Biodiversity, 11, 11.
Simmons, N.B., & Cirranello, A. L. (2024). Bat Species of the World: A taxonomic and geographic database. Version 1.5. Accessed on 02/23/2024.
Srilopan, S. (2018). Diet of the wrinkle-lipped free-tailed bat (Chaerephon plicatus Buchannan, 1800) in central Thailand. [Doctoral dissertation, Prince of Songkla University].
Taylor, P., Lim, B., Pennay, M., Soisook, P., Kingston, T., Loureiro, L., & Moras, L. (2019). Molossidae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats (pp. 598-672). Lynx Edicions.
Thong, V. D. (2014). Taxonomic and distributional assessments of Chaerephon plicatus (Chiroptera: Molossidae) from Vietnam. Academia Journal of Biology, 36(4), 479-486.
Thong, V. D., Limbert, H., & Limbert, D. (2022). First Records of Bats (Mammalia: Chiroptera) from the World’s Largest Cave in Vietnam. Diversity, 14(7), 534.
Thongjued, K., Chotigeat, W., Bumrungsri, S., & Kitpipit, T. (2018). A preliminary diet analysis of wrinkle-lipped free-tailed bat (Chaerephon plicatus, Buchannan, 1800) using direct PCR-DGGE technique. Proceedings of the International Bioscience Conference and the 7th Joint International PSU-UNS Bioscience, 114-119.
Voigt, C. C., Bumrungsri, S., & Roeleke, M. (2019). Rapid descent flight by a molossid bat (Chaerephon plicatus) returning to its cave. Mammalian Biology, 95, 15-17.
Yang, T., Hou, X., Wang, Y., & Zhou, J. (2014). Bat species diversity and conservation in Libo World Natural Heritage site of south China Karst. Biodiversity Science, 22(3), 385.
Zhang, J. S. (2010). The bats (Mammalia: Chiroptera) of China: an integrative approach to the taxonomy [Doctoral dissertation, Graduate University of the Chinese Academy of Sciences].
Hong Kong Bat Radar. (09/11/2025). A Field Guide to Bats of Hong Kong: Wrinkle-lipped Free-Tailed Bat (Mops plicatus ). https://hkbatradar.com/en/mops_plicatus