- Hong Kong Bat Radar
- Japanese Pipistrelle (Pipistrellus abramus)
Japanese Pipistrelle
Pipistrellus abramus (Temminck, 1838)
Taxonomy
| Family: | Vespertilionidae |
| Genus: | Pipistrellus |
| Scientific name: | Pipistrellus abramus (Temminck, 1838) |
| Synonyms: |
Vespertilio abramus Temminck, 1838, V. akokomuli Temminck, 1840, V. pumiloides Tomes, 1857 |
| Common name: | Japanese Pipistrelle |
| Other name: | Japanese House Bat, Javan Pipistrelle |
| Remark: | - |
| Characteristics | |
| Color: | The dorsal fur commonly appears as gray-brown/gray-olive (dark brown at the base, with tips in gray-brown or light brown), while he ventral fur is gray-white/light gray-brown (black-brown at the base, with tips in gray-white). Some individuals may have an overall brownish-yellow coloration. Juvenile bats generally have darker fur, appearing overall gray-black. |
| Ear: | The ear pinna is slightly triangular, with rounded tips. The color of the ear pinna and tragus varies in shades of brown among individuals. The tragus is thumb-shaped, short and narrow, with a rounded and slightly forward-curved tip, approximately half the length of the ear pinna. |
| Head: | The muzzle is brown in color, with varying darkness among individuals. There are slight lateral swellings on both sides of the snout, which contain glands that can secrete a brownish-yellow liquid when under stress. The facial and periocular skin generally appears flesh-colored. |
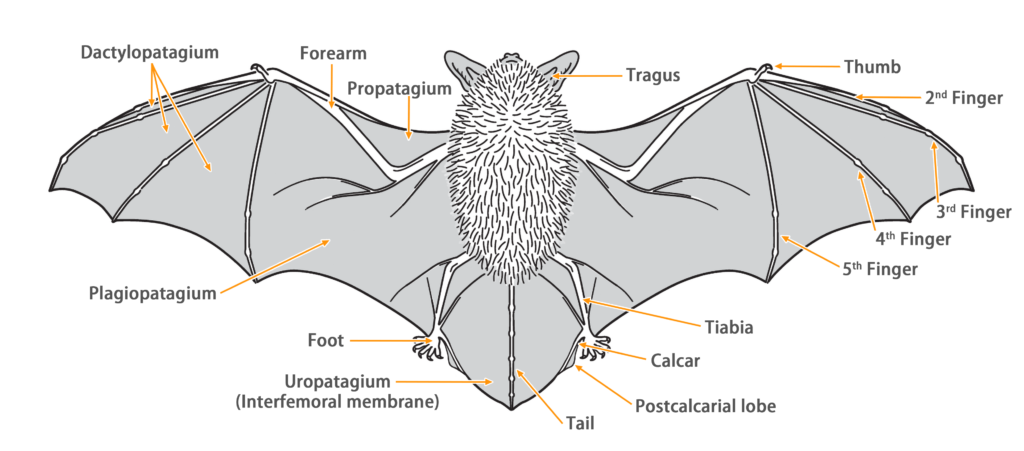
| Limbs: | The hind foot are relatively small, with the wing membrane connecting to the base of the toes. |
| Penis: | The penis is long and distinct (9.1 - 12.7 mm). The baculum is long (> 9 mm) has an S-shaped curvature. |
| Tail: | The tail is long, completely enveloped by the interfemoral membrane, with a slight protrusion (< 1 mm) at the tip of the membrane. The calcar is long, extending from the ankle to about half the length of the edge of the uropatagium, with a well-developed postcalcarial lobe. The postcalcarial lobe has a conspicuous transverse cartilaginous septum in the center. |
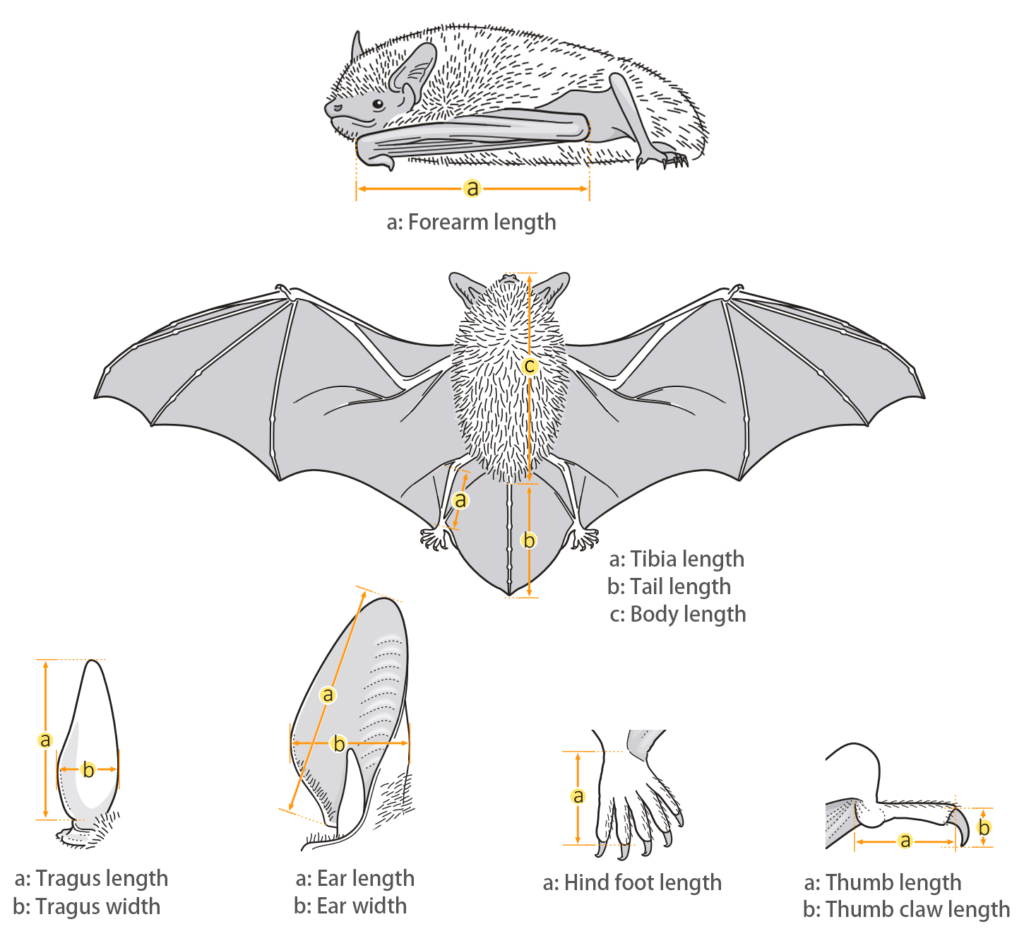
| Body measurements | |
| Size: | Small pipistrelle |
| Body: | 38.0 - 60.0 mm |
| Tail: | 27.0 - 45.0 mm |
| Ears: | 8.0 - 13.0 mm |
| Hind foot: | 6.0 - 10.0 mm |
| Forearm: | 29.0 - 36.0 mm |
| Weight: | 3.8 - 5.8 g |
| Wing morphology (Female) | |
| Wing span: | 0.109 m |
| Wing area: | 0.004 m2 |
| Wing loading: | 16.30 ± 2.00 N/m2 (High) |
| Aspect ratio: | 4.00 ± 0.30 (Low) |
| Tip-shape index: | 2.00 ± 0.50 (High) |
| Reference: | Shao et al., 2014 |
Ecology
| Habitat: | They exhibit strong adaptability and can inhabit various environments, including forests, rural areas, and urban settings. They primarily reside in crevices of buildings such as rooftops, eaves, parapets, wall holes, gaps, and window crevices, as well as narrow gaps in electrical appliances or tools (such as exhaust fans, air conditioners, sun umbrellas, and metallic pipes). They can also inhabit bat boxes. Additionally, there are records of them inhabiting caves and the nests of the Red-rumped Swallow (Cecropis daurica) in foreign locations. |
| Habit: | They have a communal roosting habit, and the size of the colony depends on the available roosting space. Individual bats that roost solitarily generally occupy narrow crevices, while larger colonies choose more spacious roosting sites such as bat boxes and parapets. The average colony consists of 20 individuals, with a recorded maximum of 250 bats in Japan. Breeding colonies during the breeding season tend to be larger, with approximately 1-110 bats in the colony when the young bats are born. Female bats typically cohabit with their young throughout the year, while male bats generally roost solitarily. |
| Reproduction: | The breeding season for local colonies occurs in May to June, although the timing varies among different colonies, with births typically occurring in the early to mid-May. The young bats are weaned by late August. Each litter consists of 2-3 offspring, but the mortality rate is high, with usually only half of the offspring surviving until weaning. After weaning, there is a mortality rate of approximately 20% for females and 90% for males during the period until one year of age. |
| Lifespan: | They have a relatively short lifespan, with males typically living 1-3 years and females 4-5 years. |
| Hibernation: | In Japanese colonies, they reduce flying and activity time when temperatures are lower (around 16-20°C). They rarely fly out at temperatures below 15°C or during heavy rainfall, and enter a hibernation period if low temperatures persist. However, in Taiwan and Hong Kong, they remain active throughout the year without hibernation, although their activity levels vary depending on weather temperatures and food availability. Local colonies in colder winter months (December to February) still fly out for foraging, but the frequency of leaving the roost and the duration of activity noticeably decrease. |
| Flight: | They have fast flight speeds, good maneuverability, and hovering ability, but their flight efficiency is relatively low, making them suited for medium-distance flights. |
| Foraging: | They are nocturnal bats, emerging from their daytime roosts approximately 10-30 minutes before dusk to forage. The timing of their flight emergence is influenced by the local light conditions, and they tend to fly out earlier on cloudy days. They have two peak foraging periods each night, occurring after sunset (lasting at least 3 hours) and before dawn (lasting at least 1 hour), during which they rest briefly in different nighttime roosts. They can be observed foraging in various open habitats, including urban parks, farmland, fish ponds, wastelands, forests, wetlands, ponds, and streams. They are accustomed to a looping flight pattern while foraging, often hovering and maneuvering around the canopy, shrubs, bodies of water, or streetlights. They have a wide-ranging activity area, and some individuals may forage near their roosts before flying to more distant foraging sites, with records of bats flying up to 5 km from their roosts for foraging. |
| Diet: | They are insectivorous bats, capturing insects in mid-air during flight. They have a diverse diet, which includes insects from 12 different orders and spiders. They adjust their foraging strategies based on changes in food availability due to habitat and seasonal factors. For example, urban colonies in Japan primarily prey on insects from the Lepidoptera, Diptera, and Hemiptera orders, while those in rice fields primarily prey on insects from the Diptera, Hemiptera, and Hymenoptera orders. |
Diet composition of P. abramus in Taiwan (Lee & Lee, 2005)
Diet composition of P. abramus in Japan (Hirai & Kimura, 2004)
Distribution
| Local: | The New Territory, Hong Kong Island and Lantau Island |
| Global: | Southwest Russian Far East (Ussuri region), North and South Korea, Japan including many offshore Islands (Tsushima, Yakushima, Tanegashima, Kuchinoshima, Takarajima, Amami-Oshima, Kakeroma-Jima, Tokunoshima, Okinawajima, Miyakojima, Irabu, Ishigakijima, Iriomotejima, and Yonagunijima), Central, East & South China, Taiwan and Hainan Islands, North Myanmar, North Laos, North & Central Vietnam (including Cat Ba and Kaitien Islands), and scattered records in North-central, South-central & Northeast India (Uttar Pradesh, Arunachal Pradesh, and Andhra Pradesh); there is a record from Sakhalin Island but this requires confirmation. (Moratelli et al., 2019) |
Local distribution map
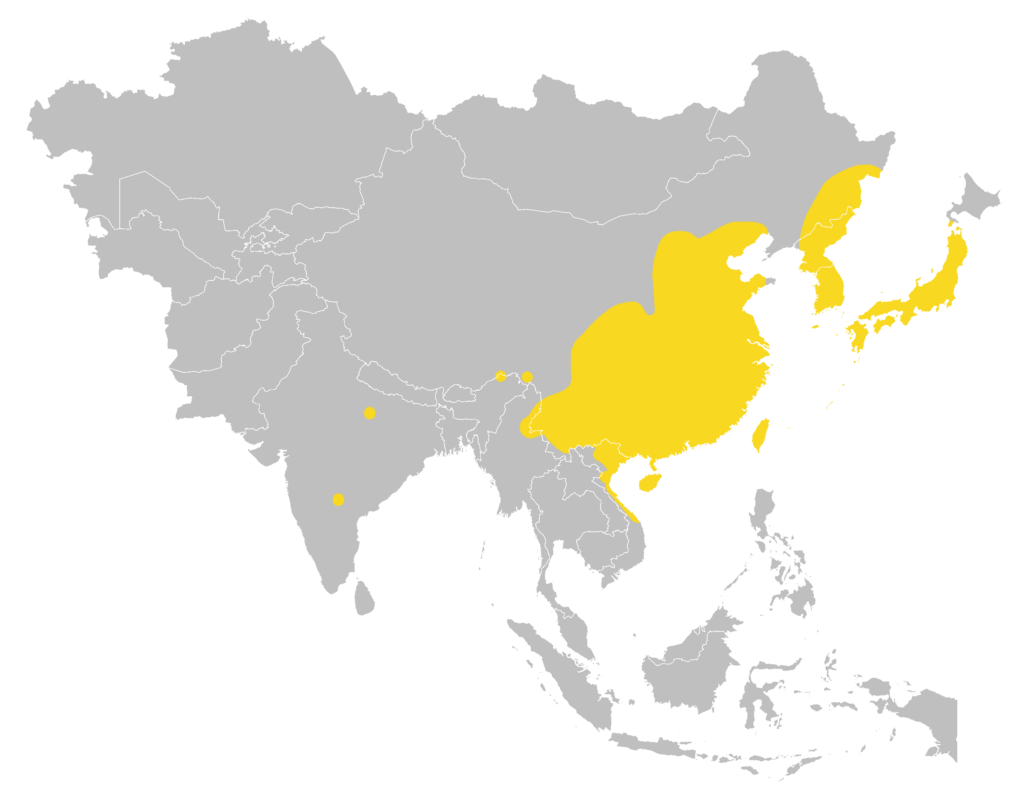
Global distribution map
(Moratelli et al., 2019)
Status and Conservation
| First record: | 1955 |
| Origin: | Native |
| Local status: | Very Common (Shek & Chan, 2005) |
| National status: | Least Concern (Red List of China Vertebrates) |
| Global status: | Least Concern (IUCN Red List) |
| Potential threat: | TBC |
Echolocation

| Parameter | Value |
|---|---|
| Call structure | FM/QCF |
| Duration | 3.50 ± 0.38 ms |
| Inter pulse interval | 46.83 ± 17.49 ms |
| Peak frequency | 58.10 ± 3.80 kHz |
| Highest frequency | 78.00 ± 5.72 kHz |
| Lowest frequency | 45.86 ± 1.57 kHz |
|
Region: |
China (Guizhou) |
| Method: | Flight tent |
| Reference: | 馮江等,2003 |
| Parameter | Value |
|---|---|
| Call structure | FM/QCF |
| Duration | 2.60 ± 0.80 ms |
| Inter pulse interval | 71.00 ± 36.50 ms |
| Peak frequency | 52.10 ± 2.40 kHz |
| Highest frequency | 86.60 ± 10.20 kHz |
| Lowest frequency | 43.40 ± 10.60 kHz |
|
Region: |
China (Wuhan) |
| Method: | Hand release |
| Reference: | Luo et al ., 2007 |
| Parameter | Value |
|---|---|
| Call structure | FM/QCF |
| Duration | 6.78 ± 7.91 ms |
| Inter pulse interval | 97.77 ± 26.36 ms |
| Peak frequency | 45.52 ± 1.09 kHz |
| Highest frequency | 62.96 ± 5.81 kHz |
| Lowest frequency | 43.53 ± 0.94 kHz |
|
Region: |
China (Beijing) |
| Method: | Hand release |
| Reference: | Ma et al ., 2010 |
| Parameter | Value |
|---|---|
| Call structure | FM/QCF |
| Duration | 3.30 ± 1.10 ms |
| Inter pulse interval | 63.70 ± 38.6 ms |
| Peak frequency | 56.30 ± 6.60 kHz |
| Highest frequency | 87.70 ± 9.80 kHz |
| Lowest frequency | 47.40 ± 4.80 kHz |
|
Region: |
China (Zhoushan) |
| Method: | Flight tent |
| Reference: | Shao et al., 2014 |
| Parameter | Value |
|---|---|
| Call structure | FM/QCF |
| Duration | 5.97 ± 1.53 ms |
| Inter pulse interval | 82.68 ± 9.49 ms |
| Peak frequency | - |
| Highest frequency | 53.30 ± 5.30 kHz |
| Lowest frequency | 46.74 ± 1.94 kHz |
|
Region: |
Taiwan |
| Method: | Wild call |
| Reference: | 趙念民,2001 |
Similar Species
Least Pipistrelle
Pipistrellus tenuis
Size:
The smallest among three.
Forearm:
25.0 - 31.0 mm
Color:
Short fur, with a uniformly brownish-black color on the back (slightly lighter at the tips).
Ears:
Triangular in shape, with a dark brown color.
Tragus:
Relatively slender, with a rounded and slightly forward-curved tip.
Snout:
Dark brown to grayish-black, with prominent lateral swellings on both sides.
Tail:
A slight protrusion (< 1 mm) at the tip.
Penis:
Relatively short in length (3.8 mm).
Japanese Pipistrelle
Pipistrellus abramus
Size:
Smaller than P. tenuis and similar to or smaller than H. pulveratus.
Forearm:
29.0 - 36.0 mm
Color:
Short fur, with a gray-brown color on the back (lighter gray-brown at the tips and darker brown at the base).
Ears:
Triangular in shape, with varying degrees of brown coloration.
Tragus:
Relatively slender, with a rounded and slightly forward-curved tip.
Snout:
Dark brown to grayish-black, with slight lateral swellings on both sides.
Tail:
A slight protrusion (< 1 mm) at the tip.
Penis:
A long and distinct penis (9.1 - 12.7 mm).
Chinese Pipistrelle
Hypsugo pulveratus
Size:
Larger than P. tenuis and similar to or larger than P. abramus.
Forearm:
32.0 - 37.0 mm
Color:
Long fur, with a dark brown-black color on the back (slightly lighter at the tips).
Ears:
Slender and triangular in shape, ranging from deep gray to black in color.
Tragus:
Relatively thick and short, with a wider base and a rounded and slightly forward-curved tip.
Snout:
Dark gray to black in color, with slight lateral swellings on both sides.
Tail:
The tail is free for last two vertebrae (~ 3 mm)
Penis:
The shortest penis (2.6 - 3.1 mm) among three.
Bibliography
Chung, C. U., Kim, S. C., Jeon, Y. S., & Han, S. H. (2017). Changes in habitat use by female Japanese Pipistrelles (Pipistrellus abramus) during different stages of reproduction revealed by radio telemetry. Journal of Environmental Science International, 26(7), 817-826.
Funakoshi, K., & Uchida, T. (1978). Studies on the physiological and ecological adaptation of temperate insectivorous bats: III. Annual activity of the Japanese house-dwelling bat, Pipistrellus abramus. Journal of the Faculty of Agriculture, Kyushu University, 23(1/2), 95-115.
Funakoshi, K., & Uchida, T. (1982). Age composition of summer colonies in the Japanese house-dwelling bat, Pipistrellus abramus. Journal of the Faculty of Agriculture, Kyushu University, 27(1/2), 55-64.
Funakoshi, K., Katahira, R., & Ikeda, H. (2009). Night-roost usage and nocturnal behavior in the Japanese house-dwelling bat, Pipistrellus abramus. Mammal study, 34(3), 131-139.
Hirai, T., & Kimura, S. (2004). Diet composition of the common bat Pipistrellus abramus (Chiroptera; Vespertilionidae), revealed by fecal analysis. Japanese Journal of Ecology, 54, 159-163.
Hughes, A. C., Satasook, C., Bates, P. J. J., Soisook, P., Sritongchuay, T., Jones, G., & Bumrungsri, S. (2011). Using Echolocation Calls to Identify Thai Bat Species: Vespertilionidae, Emballonuridae, Nycteridae and Megadermatidae. Acta Chiropterologica, 13(2), 447–455.
Jeyapraba, L., Margaret, I. V., Addline, D., & Sakthi, V. (2023). Prediction of foraging strategy of insectivorous bats through their wing morphology. Journal of Survey in Fisheries Sciences, 10(3S), 1903-1917.
Jayaraj, V. K., Tahir, N. A., Udin, N. A., Baharin, N. K., Ismail, S. K., & Zakaria, S. N. A. (2012). Species diversity of small mammals at Gunung Stong state park, Kelantan, Malaysia. Journal of Threatened Taxa, 4(6), 2617-2628.
Jiang, Z. G., Jiang, J. P., Wang, Y. Z., Zhang, E., Zhang, Y. Y., Li, L. L., Xie, F., Cai, B., Cao, L., Zheng, G. M., Dong, L., Zhang, Z. W., Ding, P., Luo, Z. H., Ding, C. Q., Ma, Z. J., Tang, S. H., Cao, W. X., Li, C. W., Hu, H. J., Ma, Y., Wu, Y., Wang, Y. X., Zhou, K. Y., Liu, S. Y., Chen, Y. Y., Li, J. T., Feng, Z. J., Wang, Y., Wang, B., Li, C., Song, X. L., Cai, L., Zang, C. X., Zeng, Y., Meng, Z. B., Fang, H. X., & Ping, X. G. (2016). Red List of China’s Vertebrates. Biodiversity Science 24(5), 500‑551.
Lee, Y. F., & Lee, L. L. (2005). Food Habits of Japanese Pipistrelles Pipistrellus abramus (Chiroptera: Vespertilionidae) in Northern Taiwan. Zoological Studies, 44(1), 95-101.
Liu, S. Y., Wu, Y., & Li, S. (2022). Handbook of the mammals of China (3rd ed.). The Straits Publishing & Distribution Group.
Luo, F., Ma, J., Li, A. A., Wu, F. J., Chen, Q. C., & Zhang, S. Y. (2007). Echolocation calls and neurophysiological correlations with auditory response properties in the inferior colliculus of Pipistrellus abramus (Microchiroptera: Vespertilionidae). Zoological Studies, 46(5), 622-630.
Ma, J., Jones, G., Zhu, G. J., & Metzner, W. (2010). Echolocation behaviours of the Japanese pipistrelle bat Pipistrellus abramus during foraging flight. Acta Theriologica, 55(4), 315-332.
Moratelli, R., Burgin, C., Cláudio, V., Novaes, R., López-Baucells, A., & Haslauer, R. (2019). Vespertilionidae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats. (pp. 716-981). Lynx Edicions.
Morii, R. (1993). Horizontal distribution of Pipistrellus abramus in Kagawa Prefecture, Japan. Kagawa-seibutu, 20, 1-5.
Shao, W. W., Wei, L., Hu, K. L., Lin, Z. H., & Zhang, L. B. (2014) Wing morphology, echolocation calls and emergence time of Pipistrellus abramus. Acta Theriologica Sinica, 34(3), 245-251.
Shek, C. T. (2006). A Field Guide to the Terrestrial Mammals of Hong Kong. Friends of country park and cosmos book limited.
Shek, C. T., & Chan, C. S. M. (2006). Mist Net Survey of Bats with Three New Bat Species Records for Hong Kong. Hong Kong Biodiversity, 11, 1-7.
Taniguchi, K., Minegishi, H., & Kinoshita, A. (1990). Ecological data on common Japanese pipistrelle (2). Memoir of Kawasaki City Museum for Youth, 1, 23-28.
Tong, C. P. (2016). Distribution and preference of landscape features and foraging sites of insectivorous bats in Hong Kong urban parks. [Master’s thesis, The University of Hong Kong].
Yasui, S., Maruyama, N., & Kanzaki, N. (1997). Roost site selection and colony size of the common Japanese Pipistrelle (Pipistrellus abramus) in Fuchu, Tokyo. Wildlife Conservation Japan, 2(2), 51-59.
馮江、劉穎、陳敏、李振新、張喜臣、周江(2003)。普通伏翼蝠(Pipistrellus abramus)迴聲定位及母嬰交流行為研究。自然科學進展,13(12),1247-1252。(In Chinese only)
趙念民(2001)。利用回聲定位叫聲特性鑑別東亞家蝠、摺翅蝠、台灣葉鼻蝠和台灣小蹄鼻蝠之研究〔碩士論文〕。國立中山大學生物科學系。(In Chinese only)
Hong Kong Bat Radar. (09/11/2025). A Field Guide to Bats of Hong Kong: Japanese Pipistrelle (Pipistrellus abramus ). https://hkbatradar.com/en/pipistrellus_abramus