- Hong Kong Bat Radar
- Andersen’s Roundleaf Bat (Hipposideros gentilis)
Andersen's Leaf-nosed Bat
Hipposideros gentilis K. Andersen, 1918
Taxonomy
| Family: | Hipposideridae |
| Genus: | Hipposideros |
| Scientific name: | Hipposideros gentilis K. Andersen, 1918 |
| Synonyms: | - |
| Common name: | Andersen's Leaf-nosed Bat |
| Other name: | Andersen's Roundleaf Bat, Exotic Leaf-nosed Bat |
| Remark: | H. gentilis has originally been classified as a subspecies of H. pomona, but recent morphological studies (Srinivasulu & Srinivasulu, 2018) have indicated that the population in South China represents a distinct species, H. gentilis. H. gentilis comprises two known subspecies: H. g. gentilis (K. Andersen, 1918) and H. g. sinensis (K. Andersen, 1918). Based on geographical distribution, Anderson's Leaf-nosed bats in Hong Kong belong to H. g. sinensis. The classification status of H. gentilis still holds many mysteries, including the distribution of its subspecies and the possible existence of hidden subspecies in China (Hainan Island). |
| Characteristics | |
| Color: | The dorsal fur and base of the ears are brown to gray-brown (gray-white at the base and brown at the tip), while the ventral fur is lighter, appearing brownish-white. Occasionally, individuals with partially whitened fur can be observed. Juvenile bats were generally darker in color, appearing gray-black. |
| Ear: | The ear pinna is broad, with multiple transverse ridges on the inner side and slightly pointed ear tips. Both the ear pinna and antitragus are brown to dark brown in color. The antitragus is short and connected to the ear shell. |
| Head: | The muzzle is brown in color, with varying darkness among individuals. There are slight lateral swellings on both sides of the snout, which contain glands that can secrete a brownish-yellow liquid when under stress. The facial and periocular skin generally appears flesh-colored. The head is small while the ears, eyes, and nose leaf are proportionally large, giving the bat an especially cute appearance. |
| Nose leaf: | The nose leaf is relatively simple and is divided into three parts: anterior, intermediate, and posterior leaf. The anterior leaf has a horseshoe shape with a slight upward curve at the central front, and it is wider than long, lacking supplementary leaflets. The intermediate leaf is not well-developed and slightly narrower than the anterior and posterior leaf. It features four evenly spaced circular glandular protrusions, each with a long hair. The posterior leaf is wide and short, similar in width to the anterior leaf. The internarial septum starts off slightly narrow at the base, then becomes parallel-sided, and gradually tapers to a blunt tip, resembling a tapered triangle that almost touches the intermediate leaf. |
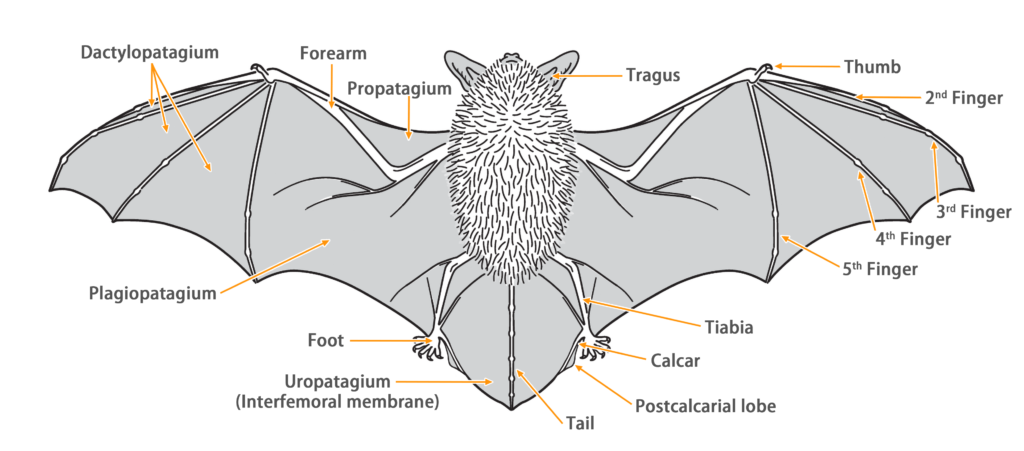
| Tail: | The tail is long, completely enveloped by the interfemoral membrane, with a slight protrusion at the tip of the membrane. |
| Other: | Behind the anterior leaf, there is a frontal sac with a ∩-shaped opening, which is usually covered with fur and not prominent. |
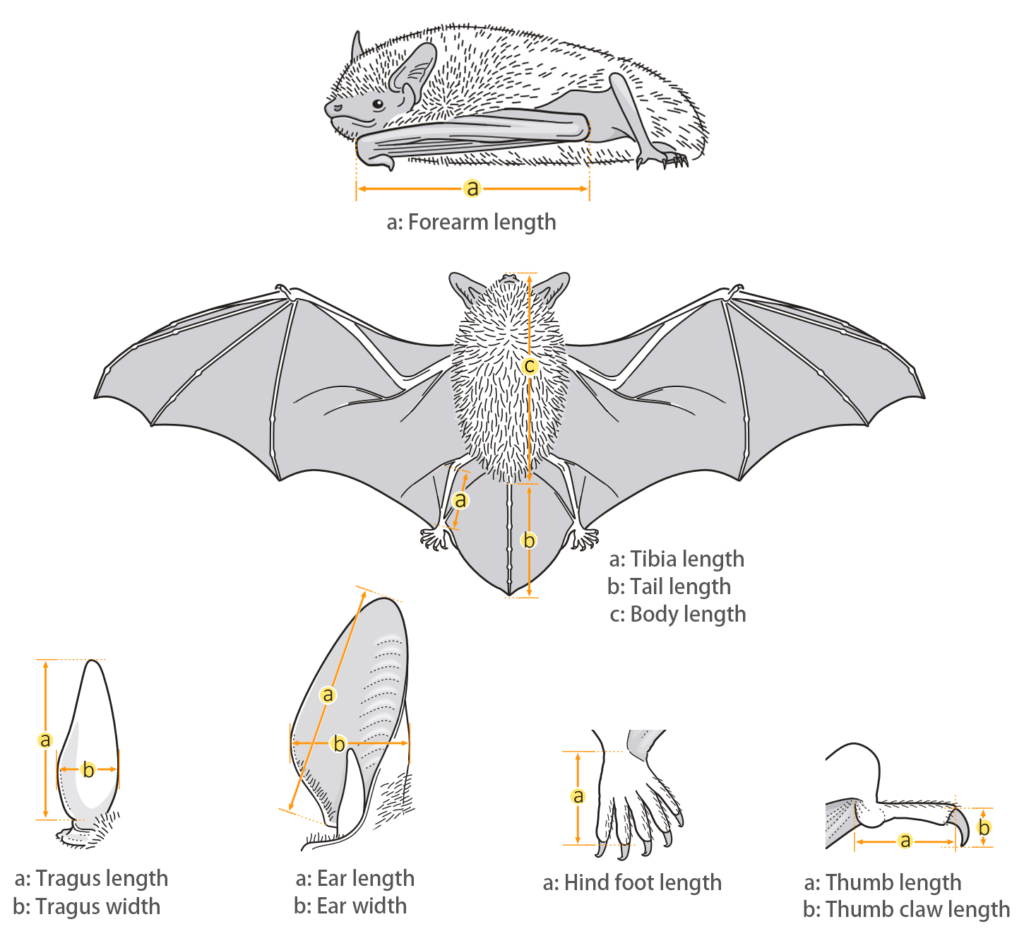
| Body measurements | |
| Size: | Small Leaf-nosed bat |
| Body: | 36.0 mm |
| Tail: | 28.0 - 35.0 mm |
| Ears: | 18.0 - 25.0 mm |
| Hind foot: | 6.0 - 9.0 mm |
| Forearm: | 38.0 - 43.0 mm |
| Weight: | 6.0 - 8.3 g |
| Wing morphology | |
| Wing span: | 0.242 m |
| Wing area: | 0.012 m2 |
| Wing loading: | 5.44 ± 0.73 N/m2 (Very low) |
| Aspect ratio: | 4.80 ± 0.28 (Low) |
| Tip-shape index: | 1.78 ± 0.27 (Medium) |
| Reference: | Furey & Racey, 2016 |
Ecology
| Habitat: | Cave-dwelling bats, which can inhabit various environments, prefer to reside in remote caves away from human activity. These caves include drainage channels, waterways, small watercourses, abandoned mines, and air-raid shelters. Some of them also inhabit sea caves and abandoned buildings. They exhibit a preference for the deep recesses or narrow spaces at the end of caves. Their habitats typically feature low ceilings, high temperature, humidity, and minimal human disturbance. During the breeding season, the reproductive colonies favor caves with abundant water and high humidity for rearing their offspring. |
| Habit: | During the winter, they form large colonies consisting of hundreds to over a thousand individuals for hibernation. The largest recorded winter colony in the local area comprised over 900 individuals (March 2004, Lin Fa Shan Mine Cave). They typically roost on the upper parts of caves or higher walls, but if the colony density becomes too high, some individuals may choose lower positions. While roosting in colonies, individuals maintain a certain distance between each other. In the summer, some individuals leave their original winter colonies and migrate to other habitats for roosting and reproduction. |
| Reproduction: | The breeding season for local colonies occurs from May to June each year, with typically one offspring being produced. The eyes of the young bat fully open on the 13th day after birth, and they are capable of short-distance flight by day 19. By day 31, they can fly with agility. Young bats often cling to the ventral side of the mother bat in an inverted position, and the mother bat carries them during foraging trips. However, sometimes the mother bat may leave capable hanging young bats in the roost to wait. In later stages, when the young bats grow to a size similar to adults, they hang on the mother bat's shoulders. |
| Hibernation: | They enter hibernation approximately from late December to February, but the actual duration of hibernation can vary depending on the temperature of the weather. |
| Flight: | Their flight speed is slow, and their flight efficiency is not remarkable. However, they possess excellent agility and can make rapid turns, making them well-suited for short-distance flights. Lesser horseshoe bats have rapid wing beats and are accustomed to slow flight in low-altitude areas or dense forests. They often pause to rest on branches and leaves. |
| Foraging: | Their hunting methods include aerial hunting, gleaning, and hawking. After capturing prey, they bring it back to their roost to consume, leaving behind the remains of the prey in the roost. |
| Diet: | They are insectivorous, and the size of their prey varies greatly, ranging from insects as small as 3mm or less in the suborder Nematocera to larger insects like the approximately 50mm long Golden moon moth (Actias sinensis). They have a particular fondness for hunting insects in the order Lepidoptera, which includes moths and butterflies, such as Lagoptera juno, Lyssa Zampa, Polyura athamas, and Melanitis leda, etc. They also prey on insects in the order Coleoptera (beetles), albeit to a lesser extent. |
Diet composition of H. gentilis in Hong Kong (Shek Kong) (Ades, 1994)
Diet composition of H. gentilis in
Hong Kong (Lin Fa Shan) (Ades, 1994)
Distribution
| Local: | The New Territory, Hong Kong Island, Lantau Island and Lamma Island |
| Global: | |
| H. g. gentilis | Nepal, Northeast India, Bangladesh, Myanmar, Thailand, Peninsular Malaysia, and Andaman Islands |
| H. g. sinensis | South & Southeast China (including Hainan Island), Thailand, Laos, Vietnam and Cambodia (Monadjem et al., 2019) |
| H. g. ssp. | China (Hainan Island) may have cyptic subspecies (Zhao et al., 2015) |
Local distribution map
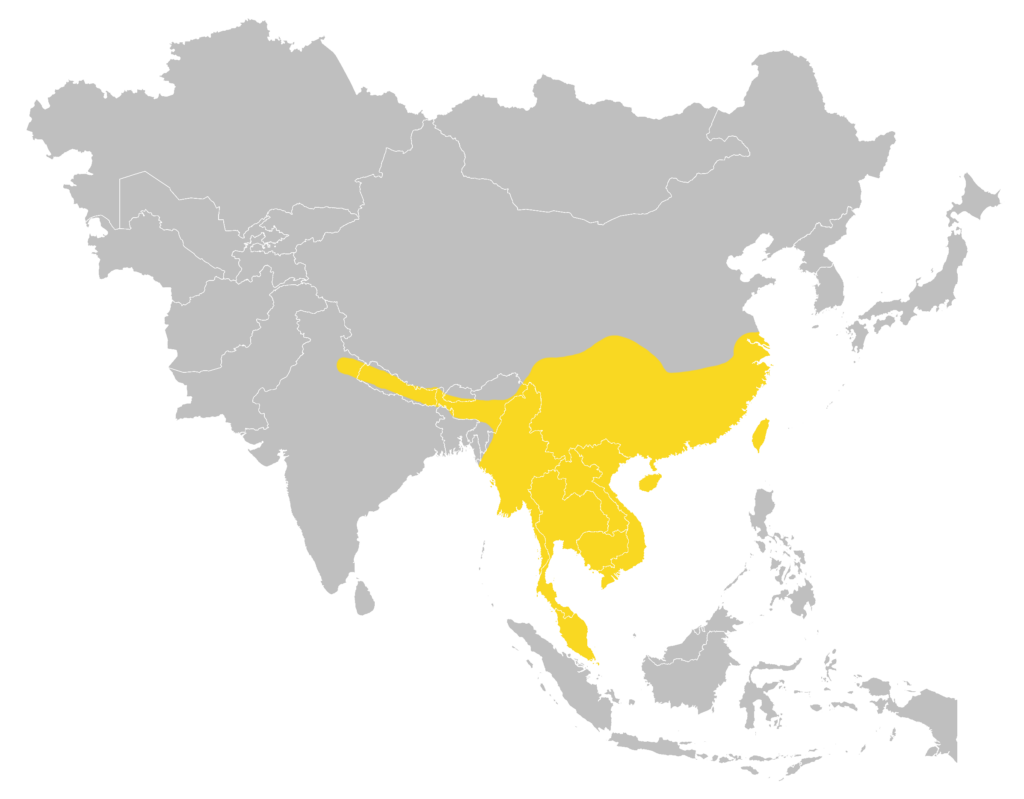
Global distribution map
(Monadjem et al., 2019)
Status and Conservation
| First record: | 1953 |
| Origin: | Native |
| Local status: | Very common (Shek & Chan, 2005) |
| National status: | Least Concern (Red List of China Vertebrates) |
| Global status: | Not Evaluated (IUCN Red List) |
| Potential threat: | TBC |
Echolocation
| Parameter | Value |
|---|---|
| Call structure | CF-FM |
| Duration | 6.30 ms |
| Inter pulse interval | 23.20 ms |
| Peak frequency | - |
| Highest frequency | 129.60 kHz |
| Lowest frequency | 103.10 kHz |
| Subspecies: | H. g. sinensis |
|
Region: |
Hong Kong |
| Method: | Hand release |
| Reference: | Shek & Lau, 2006 |
| Parameter | Value |
|---|---|
| Call structure | CF-FM |
| Duration | 7.00 ± 2.30 ms |
| Inter pulse interval | 20.90 ± 4.50 ms |
| Peak frequency | 125.10 ± 2.30 kHz |
| Initial frequency | 125.00 ± 2.50 kHz |
| End frequency | 104.10 ± 6.30 kHz |
| Subspecies: | H. g. sinensis |
|
Region: |
Vietnam |
| Method: | Hand release & Flight tent |
| Reference: | Furey et al., 2009 |
| Parameter | Value |
|---|---|
| Call structure | CF-FM |
| Duration | 4.60 ± 1.40 ms |
| Inter pulse interval | - |
| Peak frequency | 127.30 ± 2.80 kHz |
| Start frequency | - |
| End frequency | - |
| Subspecies: | H. g. sinensis |
|
Region: |
China (Yunnan) |
| Method: | Wild call |
| Reference: | Jin et al., 2015 |
Bibliography
Ades, G. W. J. (1994). A comparative ecological study of insectivorous bats (Hipposideridae, Vespertilionidae and Rhinolophidae) in Hong Kong, with special reference to dietary seasonality. [Doctoral dissertation, The University of Hong Kong].
Furey, N. M., Mackie, I. J., & Racey, P. A. (2009). The role of ultrasonic bat detectors in improving inventory and monitoring surveys in Vietnamese karst bat assemblages. Current Zoology, 55(5), 327 341.
Furey, N. M., & Racey, P. A. (2016). Can wing morphology inform conservation priorities for Southeast Asian cave bats?. Biotropica, 48(4), 545-556.
Guo, Q., Wang, J., Yang, Y., Zhang, G., Liu, W., Niu, H., & Bu, Y. (2022). Roost selection and ecology of Hipposideros pomona in China. Animal Biology, 72(3), 257-274.
Jeyapraba, L., Margaret, I. V., Addline, D., & Sakthi, V. (2023). Prediction of foraging strategy of insectivorous bats through their wing morphology. Journal of Survey in Fisheries Sciences, 10(3S), 1903-1917.
Jiang, Z. G., Jiang, J. P., Wang, Y. Z., Zhang, E., Zhang, Y. Y., Li, L. L., Xie, F., Cai, B., Cao, L., Zheng, G. M., Dong, L., Zhang, Z. W., Ding, P., Luo, Z. H., Ding, C. Q., Ma, Z. J., Tang, S. H., Cao, W. X., Li, C. W., Hu, H. J., Ma, Y., Wu, Y., Wang, Y. X., Zhou, K. Y., Liu, S. Y., Chen, Y. Y., Li, J. T., Feng, Z. J., Wang, Y., Wang, B., Li, C., Song, X. L., Cai, L., Zang, C. X., Zeng, Y., Meng, Z. B., Fang, H. X., & Ping, X. G. (2016). Red List of China’s Vertebrates. Biodiversity Science 24(5), 500‑551.
Jin, L., Yang, S., Kimball, R. T., Xie, L., Yue, X., Luo, B., … & Feng, J. (2015). Do pups recognize maternal calls in pomona leaf-nosed bats, Hipposideros pomona?. Animal Behaviour, 100, 200-207.
Lin, A. Q., Jin, L. R., Shi, L. M., Sun, K. P., Berquist, S. W., Liu, Y., & Feng, J. (2011). Postnatal development in Andersen’s leaf-nosed bat Hipposideros pomona: flight, wing shape, and wing bone lengths. Zoology, 114(2), 69-77.
Monadjem, A., Soisook, P., Thong, V. D., & Kingston, T.
(2019). Hipposideridae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats (pp. 227-258). Lynx Edicions.
Shek, C. T. (2006). A Field Guide to the Terrestrial Mammals of Hong Kong. Friends of country park and cosmos book limited.
Shek, C. T., & Chan, C. S. M. (2005). Roost Censuses of Cave Dwelling Bats of Hong Kong. Hong Kong Biodiversity, 10, 1-8.
Shek, C. T., & Lau, C. T. Y. (2006). Echolocation Calls of Five Horseshoe Bats of Hong Kong. Hong Kong Biodiversity, 13, 9-12.
Srinivasulu, B., & Srinivasulu, C. 2018. In plain sight: bacular and noseleaf morphology supports distinct specific status of Roundleaf Bats Hipposideros pomona Andersen, 1918 and Hipposideros gentilis Andersen, 1918 (Chiroptera: Hipposideridae). Journal of Threatened Taxa, 10(8): 12018-12026.
Tong, C. P. (2016). Distribution and preference of landscape features and foraging sites of insectivorous bats in Hong Kong urban parks. [Master’s dissertation, The University of Hong Kong].
Yuzefovich, A. P., Artyushin, I. V., & Kruskop, S. V. (2021). Not the cryptic species: diversity of Hipposideros gentilis (Chiroptera: Hipposideridae) in Indochina. Diversity, 13(5), 218.
Zhao, L. Z., Bu, Y. Z., Zhou, H. X., Zhou, H. W., Zhang, Z. X., & Niu, H. X. (2015). Differences in Hipposideros pomona from three geographical regions in China based on morphology and molecular sequences data. Journal of Mammalogy, 96(6), 1305-1316.
Hong Kong Bat Radar. (18/05/2025). A Field Guide to Bats of Hong Kong: Andersen’s Leaf-nosed Bat (Hipposideros gentilis ). https://hkbatradar.com/en/hipposideros_gentilis