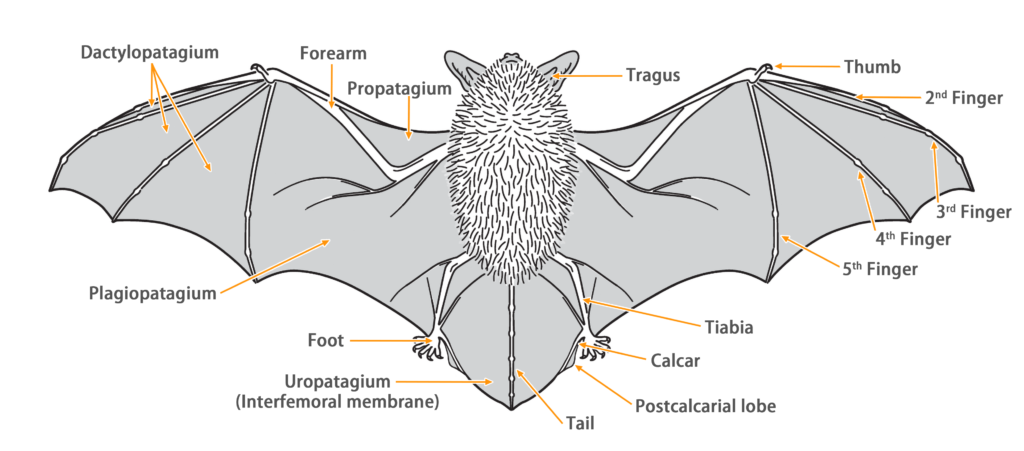
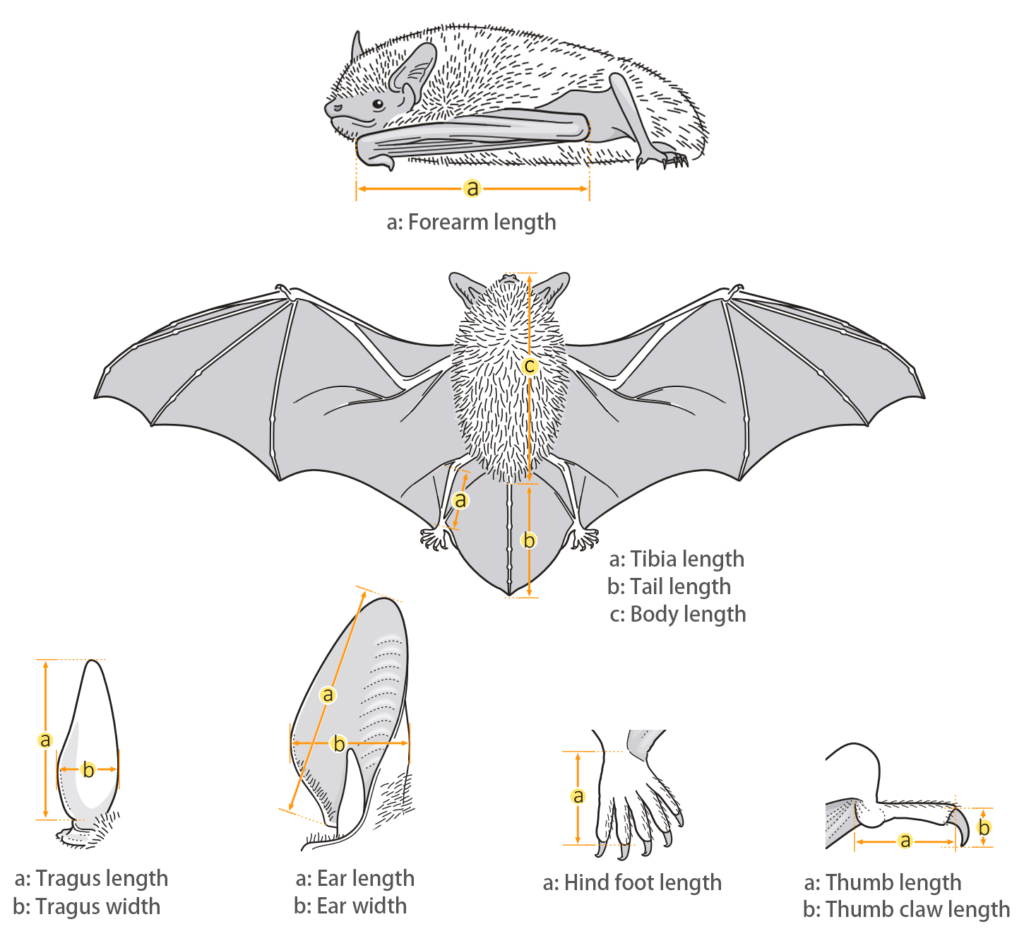
Taxonomy
| Family: | Vespertilionidae |
| Genus: | Myotis |
| Scientific name: | Myotis chinensis (Tomes, 1857) |
| Synonyms: | - |
| Common name: | Chinese Myotis |
| Other name: | Large Myotis |
| Remark: | Myotis chinensis was formerly classified as a subspecies of Myotis myotis, but it is evident from both morphological and molecular genetic evidence that Myotis chinensisis a distinct and independent species. |
| Characteristics | |
| Color: | The dorsal fur is dark brownish-gray, while the ventral fur is lighter, ranging from pale gray to grayish-white, with dark gray to blackish hair bases. Juvenile bats generally have darker overall fur coloration. |
| Ear: | The ears are large and slender. Both the ear pinna and tragus are dark brownish-gray, with lighter coloration at the base. The tragus is elongated and conical, slightly tilted forward, measuring approximately half the length of the ear pinna. |
| Head: | The muzzle and face are dark brown with short dark gray fur, while the skin around the eyes appears reddish-brown or pink. The face possesses glands that can secrete reddish-brown substances when under pressure (sometimes these secretions may also appear on the inner side of the ears). |
| Limbs: | The hind feet are large, and the claws have pigmentation. The calcar is long and unkeeled. |
| Wing: | The wing membrane attaches to the ankle or the middle of tibia. |
| Tail: | The tail is long and entirely enveloped by the interfemoral membrane. The calcar is unkeeled and over halfway to tail from ankle. |
| Body measurements | |
| Size: | Large myotis |
| Body: | 91.0 - 97.0 mm |
| Tail: | 53.0 - 58.0 mm |
| Ears: | 20.0 - 23.0 mm |
| Hind foot: | 16.0 - 18.0 mm |
| Forearm: | 64.0 - 69.0 mm |
| Weight: | 25.0 - 30.0 g |
| Wing morphology | |
| Wing span: | 0.426 m |
| Wing area: | 0.033 m2 |
| Wing loading: |
9.53 ± 0.77 N/m2 (Mid) |
| Aspect ratio: | 5.46 ± 0.12 (Low) |
| Tip-shape index: | 1.21 ± 0.23 (Low) |
| Reference: | Jennings et al., 2004 |
Ecology
| Habitat: | Typically found inhabiting large abandoned mine tunnels, they commonly take refuge in crevices within the tunnel walls and tend to occupy locations closer to the entrance of the cave. |
| Habit: | They are typically solitary, occasionally forming small colonies consisting of 2 to 10 individuals. |
| Reproduction: | The breeding period for this species occurs from May to June each year, typically producing a single offspring. M. chinensis forms large reproductive colonies with the M. pilosus, Miniopterus magnater and Miniopterus pusillus. Within these colonies, M. chinensis tends to occupy the peripheral areas. |
| Hibernation: | They undergo hibernation typically from late December to February, with the actual duration of hibernation varying in response to temperature fluctuations |
| Flight: | They exhibit a moderate flight speed and sufficient agility, although their flight efficiency and aerial hovering capabilities are relatively poor, making them more suitable for short-distance flights. They typically fly at lower altitudes, often in close proximity to the ground. |
| Foraging: | This species exhibits crepuscular activity, with two peaks of activity occurring in the evening and before dawn, while resting in the roost during the intervening period. |
| Diet: | They are insectivorous, primarily preying on larger insects. They are capable of capturing ground-dwelling insects through picking behavior and show a particular preference for beetles (Carabidae), grasshoppers, and flies. |
Diet composition of M. chinensis in Beijing, China (Ma et al., 2007)
Distribution
| Local: | The New Territory and Lantau Island |
| Global: | Central & SouthEast China (from Shanxi and Jiangsu South to Yunnan, Guanxi, and Guangdong; isolated records in Nei Mongol, Beijing, and Hainan Island), East Myanmar, North Thailand, North Laos, and North & Central Vietnam. (Moratelli et al., 2019) |
Local distribution map

Global distribution map
(Moratelli et al., 2019)
Status and Conservation
| First record: | 1964 |
| Origin: | Native |
| Local status: | Uncommon (Shek & Chan, 2005) |
| National status: | Near Threatened (Red List of China Vertebrates) |
| Global status: | Least Concern (IUCN Red List) |
| Potential threat: | TBC |
Echolocation

| Parameter | Value |
|---|---|
| Call structure | FM |
| Duration | 3.60 ± 0.90 ms |
| Inter pulse interval | 81.00 ± 15.30 ms |
| Peak frequency | 49.00 ± 5.60 kHz |
| Start frequency | 83.60 ± 4.30 kHz |
| End frequency | 25.20 ± 3.50 kHz |
|
Region: |
Vietnam |
| Method: | Hand release |
| Reference: | Furey et al., 2009 |
| Parameter | Value |
|---|---|
| Call structure | FM |
| Duration | 6.30 ms |
| Inter pulse interval | 53.90 ms |
| Peak frequency | 54.20 kHz |
| Start frequency | 83.60 kHz |
| End frequency | 14.00 kHz |
|
Region: |
China (Beijing) |
| Method: | Flight tent |
| Reference: | Ma et al., 2007 |
Similar Species
Rickett's Big-footed Myotis
Myotis pilosus
Size:
Medium myotis
Color:
The dorsal fur is light grayish-brown, while the ventral fur is grayish-white to white.
Head:
The muzzle and face are lighter in color, typically appearing pinkish-gray
Tragus:
Slender, elongated, and pointed, overall relatively small in size.
Hindfoot:
Exceptionally large feet, measuring about 80% of the length of the tibia. The claw tips are sharp, curved, and white in color.
Wing:
The wing membranes attach to the ankle.
Behavior:
At rest, they typically face the wall and keep their body as close to it as possible; wings are usually slightly opened, covering the abdomen.
Chinese Myotis
Myotis chinensis
Size:
Large myotis
Color:
The dorsal fur is dark grayish-brown, while the ventral fur ranges from light gray to grayish-white.
Head:
The muzzle and face are darker in color, typically appearing brown to dark brown.
Tragus:
Slender, elongated, and conical, overall narrower in shape.
Hindfoot:
Large feet with pigmented claws.
Wing:
The wing membranes attach to the base of the toes.
Behavior:
It exhibits a more stable behavior and tends to remain in place when startled, huddling in a corner, rarely taking flight.
Bibliography
Furey, N. M., Mackie, I. J., & Racey, P. A. (2009). The role of ultrasonic bat detectors in improving inventory and monitoring surveys in Vietnamese karst bat assemblages. Current Zoology, 55 (5), 327 341.
Jennings, N. V., Parsons, S., Barlow, K. E., & Gannon, M. R. (2004). Echolocation calls and wing morphology of bats from the West Indies. Acta Chiropterologica, 6 (1), 75-90.
Ma, J., Liang, B., Zhang, S., & Metzner, W. (2007). Dietary composition and echolocation call design of three sympatric insectivorous bat species from China. Ecological Research, 23 (1), 113–119.
Moratelli, R., Burgin, C., Cláudio, V., Novaes, R., López-Baucells, A., & Haslauer, R. (2019). Vespertilionidae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats. (pp. 716-981). Lynx Edicions.
Shek, C. T. (2006). A Field Guide to the Terrestrial Mammals of Hong Kong. Friends of country park and cosmos book limited.
Shek, C. T., & Chan, C. S. M. (2005). Roost Censuses of Cave Dwelling Bats of Hong Kong. Hong Kong Biodiversity, 10 : 1-8.
Tong, C. P. (2016). Distribution and preference of landscape features and foraging sites of insectivorous bats in Hong Kong urban parks. [Master’s thesis, The University of Hong Kong].
Hong Kong Bat Radar. (09/11/2025). A Field Guide to Bats of Hong Kong: Chinese Myotis (Myotis chinensis ). https://hkbatradar.com/en/myotis_chinensis