- Hong Kong Bat Radar
- Least Horseshoe Bat (Rhinolophus pusillus)
Least Horseshoe Bat
Rhinolophus pusillus Temminck, 1834
Taxonomy
| Family: | Rhinolophidae |
| Genus: | Rhinolophus |
| Scientific name: | Rhinolophus pusillus Temminck, 1834 |
| Synonyms: | - |
| Common name: | Least Horseshoe Bat |
| Other name: | - |
| Remark: | R. pusillus includes nine known sub-species: R. p. pusillus (Temminck, 1834), R. p. blythi (K. Andersen, 1918), R. p. calidus (G. M. Allen, 1923), R. p. gracilis (K. Andersen, 1905), R. p. lakkhanae (Yoshiyuki, 1990), R. p. minutillus (G. S. Miller, 1906), R. p. pagi (Tate & Archbold, 1939), R. p. parcus (G. M. Allen, 1928), R. p. szechwanus (K Andersen, 1918). According to geographic distribution, the local species is R. p. calidus. |
| Characteristics | |
| Color: | Dorsal fur exhibits a wide range of variations, ranging from brownish-yellow to deep brown. Ventral fur is relatively lighter in color. Juvenile bats generally have a darker overall fur color, appearing gray-black. |
| Ear: | Ear pinna are broad and large, with a smooth outer margin and a pointed tip. The antitragus is broad and short. Both the ear pinna and antitragus are deep gray to gray-black in color. |
| Head: | Small eyes. The nasal region is specialized, featuring complex nose leaf structures. The lancet is either equilateral or elongated with concave sides and a rounded apex. The connective process forms a pointed elongated triangle with its apex pointing forward in lateral view. The sella exhibits concave sides and a rounded apex. The horseshoe is wide (6.0 - 8.0 mm) in size. |
| Mouth: | Lower lip has 3 mental grooves |
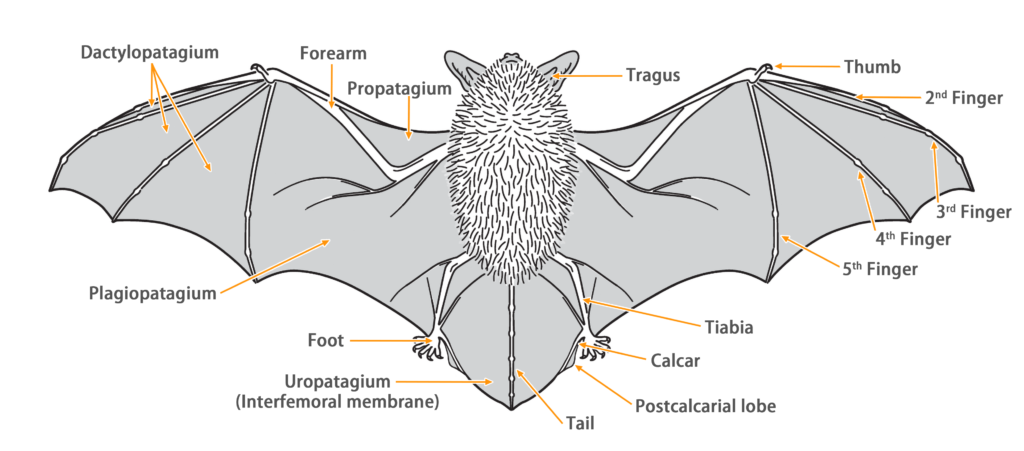
| Limbs: | Wings are attached to the ankle |
| Tail: | Long and enclosed by tail membrane |
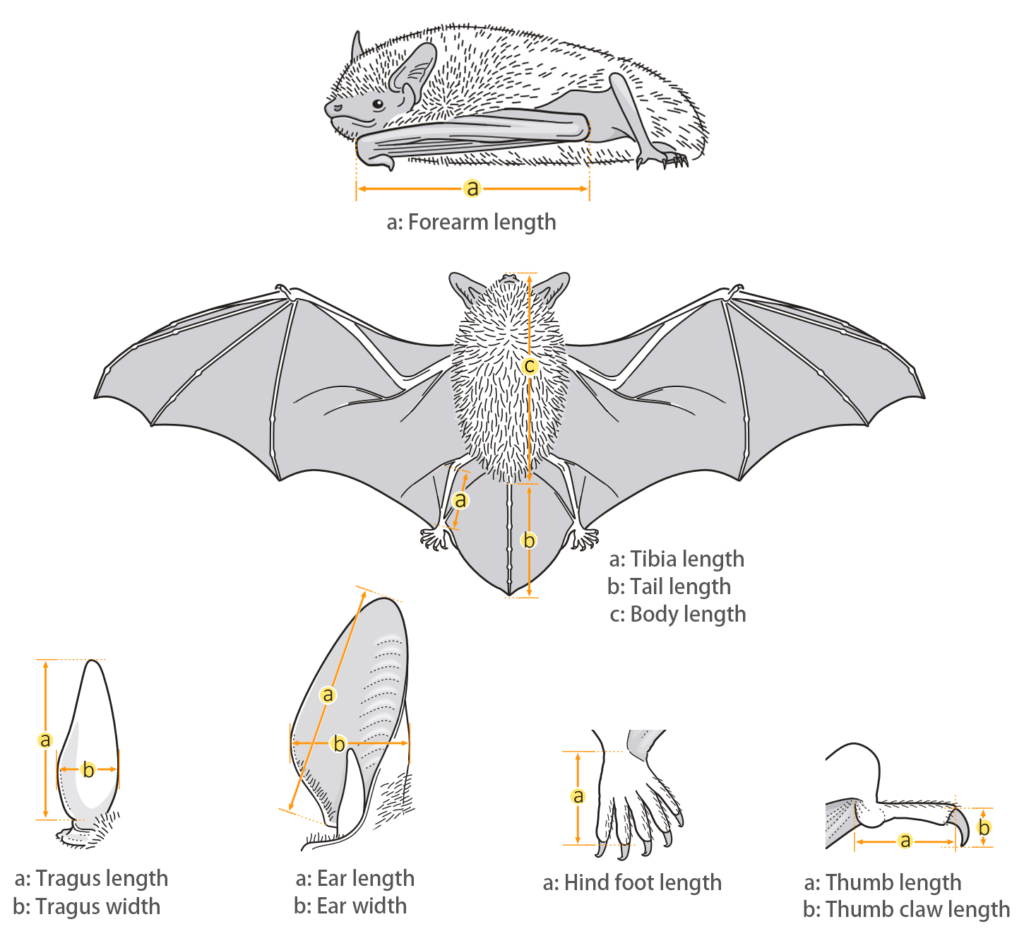
| Body measurements | |
| Size: | Small horseshoe bat |
| Body: | 30.0 - 42.0 mm |
| Tail: | 13.0 - 26.0 mm |
| Ears: | 13.0 - 20.0 mm |
| Hind foot: | 6.0 - 8.0 mm |
| Forearm: | 33.0 - 41.6 mm |
| Weight: | 3.0 - 3.8 mm |
| Wing morphology | |
| Wing span: | 0.217 m |
| Wing area: | 0.009 m2 |
| Wing loading: |
4.68 ± 0.42 N/m2 (Very Low) |
| Aspect ratio: | 5.09 ± 0.26 (Low) |
| Tip-shape index: | 1.98 ± 0.22 (High) |
| Refernce: | Furey & Racey, 2016 |
Ecology
| Habitat: | Cave-dwelling bats that can inhabit various environments, typically found in temperature-stable irrigation channels and abandoned mines. |
| Habit: | They exhibit both solitary and group roosting habits, with group sizes ranging from a few individuals to several hundred (a recorded colony in Guilin City consisted of approximately 800 bats), but local colonies tend to be smaller, with a recorded size of 46 bats. They are usually scattered on the walls of caves and occasionally reside in smaller drainage channels. |
| Reproduction: | Mating occurs from September to November, while the birthing period is from May to June, typically producing one offspring. The young bats begin weaning around 35 days after birth and become capable of independent flight and foraging. |
| Hibernation: | Hibernate from late December to February but the actual hibernation period varies according to the prevailing temperature and weather patterns. |
| Flight: | They have a relatively slow flight speed and lower flight efficiency, but possess excellent maneuverability and hovering ability, making them suitable for short-distance flights. |
| Foraging: | They are nocturnal bats, typically leaving their roosting sites within about 15 minutes after sunset and returning approximately 30 minutes before sunrise. They prefer low-level flight, often maneuvering and hunting between 1-2 meters above the ground among tree trunks, vegetation, forest paths, or streams. Sometimes, they hang on branches to rest or wait for prey. |
| Diet: | As insectivorous bats, they employ various hunting methods, including aerial feeding, gleaning and flycatching. They have a preference for feeding on insects from the orders Coleoptera, Lepidoptera, and Diptera. As insectivorous bats, they employ various hunting methods, including aerial hawking, gleaning, and flycatching. They have a preference for feeding on insects from the orders Coleoptera, Diptera, and Lepidoptera. |
Diet composition in Cambodia (Sin et al., 2020)
Diet composition in China (Guilin) (Wai et al., 2006)
Diet composition in China (Dai et al., 2023)
Distribution
| Local: | The New Territory, Hong Kong Island and Lantau Island |
| Global: | |
| R. p. pusillus : | North Sumatra, North, East & South Borneo (including Banggi I), West Java, and Bali Island. |
| R. p. blythi : | North India (Uttarakhand, Sikkim, West Bengal, Assam, Meghalaya, and Arunachal Pradesh), Nepal, Bhutan, and Southeast Bangladesh. |
| R. p. calidus : | Southeast China (Guizhou, Guangxi, Guangdong, and Fujian, along with a recent record from Beijing area that may represent this subspecies). |
| R. p. gracilis : | South India (Andhra Pradesh, Karnataka, Kerala, and Tamil Nadu). |
| R. p. lakkhanae : | South China (Yunnan), Thailand, Laos, Vietnam (including Cat Ba Island), Cambodia, and Peninsular Malaysia (including Tioman Island). |
| R. p. minutillus : | Anambas Islands (Siantan). |
| R. p. pagi : | Mentawai Islands (North Pagai). |
| R. p. parcus : | Hainan Island, China. |
| R. p. szechwanus : | Myanmar and Central China (Sichuan, Guizhou, Hubei, and probably Yunnan). (Csorba et al., 2019) |
Local distribution map
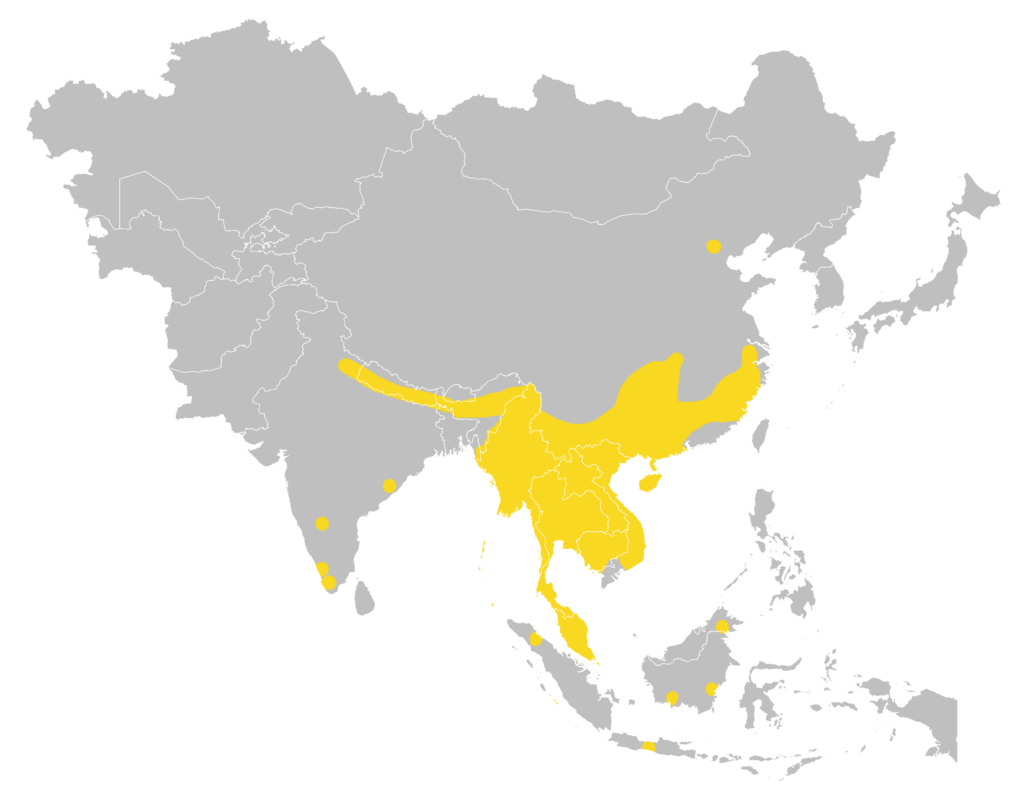
Global distribution map
(Csorba et al., 2019)
Status and Conservation
| First record: | 1964 |
| Origin: | Native |
| Local status: | Uncommon (Shek & Chan, 2005) |
| National status: | Least concern (Red List of China Vertebrates) |
| Global status: | Least concern (IUCN Red List) |
| Potential threat: | TBC |
Echolocation

| Parameter | Value |
|---|---|
| Call structure | aFM-CF-dFM |
| Duration | 32.3 (12.4 - 55.0) ms |
| Inter pulse interval | 58.6 (24.5 - 88.3) ms |
| Peak frequency | 105.4 (100.5 - 107.8) kHz |
| Highest frequency | - kHz |
| Lowest frequency | 81.7 (79.6 - 89.6) kHz |
|
Subspecies: |
R. p. calidus |
|
Region: |
Hong Kong |
| Method: | Hand release / Wild Call |
| Reference: | Shek & Lau, 2006 |
| Parameter | Value |
|---|---|
| Call structure | aFM-CF-dFM |
| Duration | - ms |
| Inter pulse interval | - ms |
| Peak frequency | - kHz |
| Highest frequency | - kHz |
| Lowest frequency | - kHz |
|
Subspecies: |
R. p. calidus |
|
Region: |
Hong Kong |
| Method: | Hand release |
| Reference: | TBC |
Similar Species
Least Horseshoe Bat
Rhinolophus pusillus
Size:
The smallest among the three.
Color:
Varies from brownish-yellow to dark brown
Lancet:
Triangular in shape with equal sides or elongated, with concave sides and a rounded blunt tip.
Connecting process:
Triangular in shape, pointing forward at the tip.
Sella:
Slightly concave on both sides, and pandurate in shape
Posture:
At rest, typically face the wall, keeping their bodies as close to the wall as possible. The wings are slightly open, covering the abdomen.
Habit:
Generally scattered in their habitat. When in groups, individuals tend to maintain a small distance between each other.
Chinese Horseshoe Bat
Rhinolophus sinicus
Size:
Intermediate between the intermediate and least horseshoe bats
Color:
Generally more vibrant, ranging from orange, rusty yellow to brownish-yellow in color.
Lancet:
Short triangular shape with a sharp tip and concave sides.
Connecting process:
Smooth and rounded on the sides.
Sella:
Parallel sides, round and without concavities, with a rounded blunt end.
Posture: At rest, the wings are usually folded and held on both sides, exposing the abdomen.
Habit:
When in groups, they form colonies with a moderate level of density and closeness.
Intermediate Horseshoe Bat
Rhinolophus affinis
Size:
The largest among the three.
Color:
Varies from brownish-yellow to dark brown
Lancet:
Long and pointed triangular shape, with a sharp and slightly forward-curved tip, and concave sides.
Connecting process:
Smooth and rounded on the sides.
Sella:
Parallel sides, with a flat and slightly concave end in the center.
Posture:
At rest, the wings are slightly open, covering the abdomen.
Habit:
When in groups, individuals usually maintain a small distance between each other.
Bibliography
Borissenko, A. V., & Kruskop, S. V. (2003). Bats of Vietnam and adjacent territories. an identification manual. Geos, Moscow, Russia.
Csorba, G., Hutson, A., Rossiter, S., & Burgin, C. (2019). Hipposideridae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats (pp. 280-332). Lynx Edicions.
Dai, W., Li, A., Chang, Y., Liu, T., Zhang, L., Li, J., Leng, H., Li, Z., Jin, L., Sun, K., & Feng, J. (2023). Diet composition, niche overlap and partitioning of five sympatric rhinolophid bats in Southwestern China during summer. Frontiers in Ecology and Evolution, 11, 1108514.
Furey, N. M., & Racey, P. A. (2016). Can wing morphology inform conservation priorities for Southeast Asian cave bats?. Biotropica, 48(4), 545-556.
Hughes, A. C., Satasook, C., Bates, P. J. J., Soisook, P., Sritongchuay, T., Jones, G., & Bumrungsri, S. (2011). Using Echolocation Calls to Identify Thai Bat Species: Vespertilionidae, Emballonuridae, Nycteridae and Megadermatidae. Acta Chiropterologica, 13(2), 447–455.
Jayaraj, V. K., Tahir, N. A., Udin, N. A., Baharin, N. K., Ismail, S. K., & Zakaria, S. N. A. (2012). Species diversity of small mammals at Gunung Stong state park, Kelantan, Malaysia. Journal of Threatened Taxa, 4(6), 2617-2628.
Jiang, Z. G., Jiang, J. P., Wang, Y. Z., Zhang, E., Zhang, Y. Y., Li, L. L., Xie, F., Cai, B., Cao, L., Zheng, G. M., Dong, L., Zhang, Z. W., Ding, P., Luo, Z. H., Ding, C. Q., Ma, Z. J., Tang, S. H., Cao, W. X., Li, C. W., Hu, H. J., Ma, Y., Wu, Y., Wang, Y. X., Zhou, K. Y., Liu, S. Y., Chen, Y. Y., Li, J. T., Feng, Z. J., Wang, Y., Wang, B., Li, C., Song, X. L., Cai, L., Zang, C. X., Zeng, Y., Meng, Z. B., Fang, H. X., & Ping, X. G. (2016). Red List of China’s Vertebrates. Biodiversity Science 24(5), 500‑551.
Shek, C. T. (2006). A Field Guide to the Terrestrial Mammals of Hong Kong. Friends of country park and cosmos book limited.
Shek, C. T., & Chan, C. S. M. (2005). Roost Censuses of Cave Dwelling Bats of Hong Kong. Hong Kong Biodiversity, 10, 1-8.
Shek, C. T., & Lau, C. T. Y. (2006). Echolocation Calls of Five Horseshoe Bats of Hong Kong. Hong Kong Biodiversity, 13, 9-12.
Sin, S., Chhorn, S., Doeurk, B., Hak, K., Ith, S., Phauk, S., & Sor, R. (2020). Diet preferences of insectivorous bats (Mammalia. Cambodian Journal of Natural History, 2020(2), 69-77.
Thong, V. D., Denzinger, A., Sang, N. V., Huyen, N. T. T., Thanh, H. T., Loi, D. N., Nha, P. V., Viet, N. V., Tien, P. D., Tuanmu, M.-N., Huang, J. C.-C., Thongphachanh, L., Luong, N. T., & Schnitzler, H. U. (2021). Bat Diversity in Cat Ba Biosphere Reserve, Northeastern Vietnam, A Review with New Records from Mangrove Ecosystem. Diversity, 13(8), 376.
Tong, C. P. (2016). Distribution and preference of landscape features and foraging sites of insectivorous bats in Hong Kong urban parks. [Master’s dissertation, The University of Hong Kong].
Wei, L., Ru, B. H., Zhou, Y. Y., Shao, W. W., Zhang, L. B., Hong, T., Zhou, S. Y., & Ma, J. (2009). Postnatal Development of Morphological Features and Vocalization of Rhinolophus pusillus. Zoological Research, 30(1), 91-98.
Wei, L., Zhou, S. Y., Zhang, L. B., Liang, B., Hong, T. Y., & Zhang, S. Y. (2006). Characteristics of echolocation calls and summer diet of three sympatric insectivorous bats species. Zoological Research, 27(3), 235.
Hong Kong Bat Radar. (09/11/2025). A Field Guide to Bats of Hong Kong: Least Horseshoe Bat (Rhinolophus pusillus ). https://hkbatradar.com/en/rhinolophus_pusillus