- Hong Kong Bat Radar
- Black-bearded Tomb Bat (Taphozous melanopogon)
Black-bearded Tomb Bat
Taphozous melanopogon Temminck, 1841
Taxonomy
| Family: | Emballonuridae |
| Genus: | Taphozous |
| Scientific name: | Taphozous melanopogon Temminck, 1841 |
| Synonyms: | Taphozous solifer Hollister, 1913 |
| Common name: | Black-bearded Tomb Bat |
| Other name: | - |
| Remark: | - |
| Characteristics | |
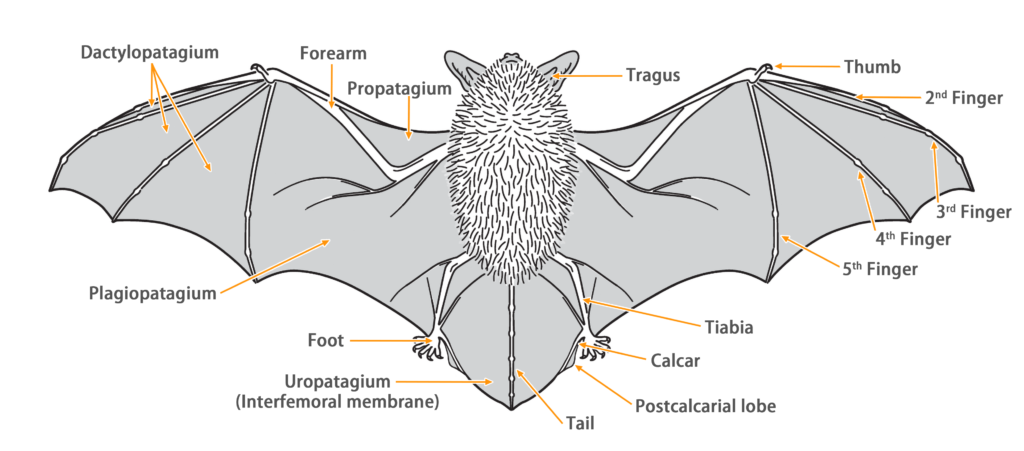
| Color: | Their pelage color varies with sex, age, and season. The dorsal fur is generally grayish-brown to brown (with white or grayish-white bases and grayish-brown to brown tips), while the ventral fur is paler, appearing light grayish-brown (with white bases, grayish-brown middles, and paler tips towards the ends). Juveniles or young adults have an overall darker pelage. |
| Ear: | The pinnae are large and triangular with numerous wrinkles on the upper half and fur on the posterior margin. The tragus is short, rounded, and axe-shaped. Both the pinna and tragus are deep brown in color. |
| Head: | The muzzle is slightly elongated, with exposed deep brown skin and sparse facial fur. The eyes are relatively large. Males develop a small tuft of black beard (up to 25 mm long) on the chin approximately 6 months after birth, with small glands (without a gular sac) under the chin, which secrete a thick secretion which runs over the beard during the period of rut. |
| Limbs: | The dorsal fur extends to approximately one-third of the proximal humerus and femur. A triangular membrane (radio-metacarpal pouch) is well-developed between the radius and fifth digit. The wing membrane attaches to the tibia above the ankle. |
| Tail: | The base of tail is thick, with the distal half (4 caudal vertebrae) protruding from the uropatagium, gradually thickening towards the slightly swollen tip with sparse hairs. The calcar is well-developed, extending from the ankle to about half the length of the trailing edge of the uropatagium (approximately 19 mm), without a keel. During flight, the uropatagium can be folded inwards. |
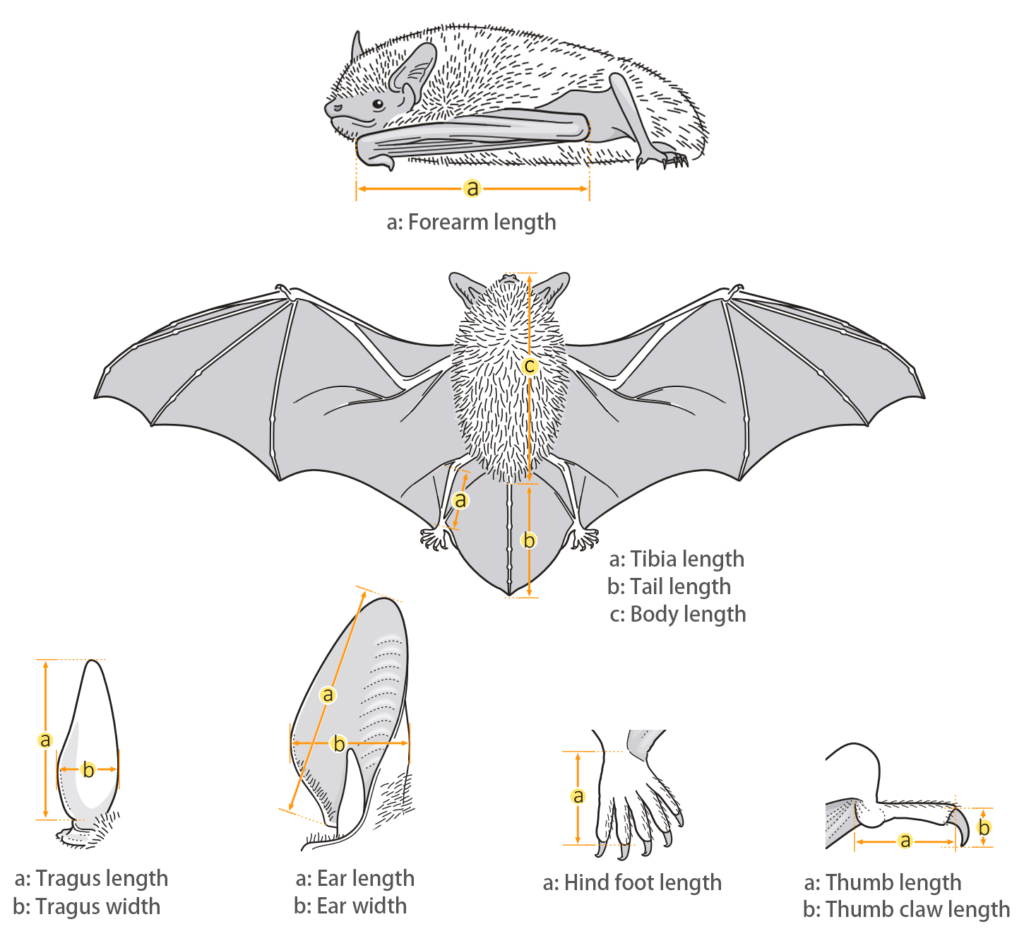
| Body measurements | |
| Size: | Small Tomb Bat |
| Body: | 67.0 - 86.0 mm |
| Tail: | 11.9 - 26.0 mm |
| Ears: | 21.0 - 23.0 mm |
| Hind foot: | 13.0 - 15.0 mm |
| Forearm: | 60.0 - 68.0 mm |
| Weight: | 21.0 - 26.0 g |
| Wing morphology | |
| Wing span: | 0.406 m |
| Wing area: | 0.019 m2 |
| Wing loading: | 13.9 ± 0.5 N/m2 (High) |
| Aspect ratio: | 9.0 ± 0.4 (High) |
| Tip-shape index: | 0.67 ± 0.09 (Low) |
| Reference: | Jeyapraba, 2023 |
Ecology
| Habitat: | Cave-dwelling bats with high adaptability, capable of inhabiting various habitats, including forested and urban areas. They typically roost in well-lit areas of caves or man-made structures, such as sea caves, large rock crevices, dilapidated buildings, castle dungeons, temples, abandoned mines, tunnels, and churches. |
| Habit: | They are gregarious with colony sizes ranging from a few individuals to thousands (e.g., a breeding colony of approximately 5,000 individuals in an old castle ruin in Burhanpur District, India). Males are territorial, with females generally clustering around them. Locally, no roost sites have been recorded, with only scattered individual records, mostly of individuals that have accidentally entered buildings. |
| Reproduction: | Based on breeding colonies in India and the Philippines, the mating period occurs during the transition from winter to spring (approximately January to February), when males and females migrate to breeding sites for mating. Males become highly territorial during the mating season and secrete a viscous substance (likely containing female-attracting pheromones) from their throats. Their mating system is polygynous, with males mating with multiple females within their territories, while females mate with a single male. After successful copulation, females immediately become pregnant, with a gestation period of approximately 125-130 days, giving birth to a single pup in late May. The nursing period lasts approximately 8 weeks, after which the females leave, and the juveniles continue their development independently. Males do not participate in the rearing process. |
| Hibernation: | They undergo hibernation, with the actual duration varying depending on temperature conditions. |
| Migration: | Before the breeding season, adults migrate to breeding sites in preparation for mating. After the breeding season concludes, they leave the breeding site, leaving the independently capable juveniles behind to continue their development. |
| Flight: | Their wings are narrow and elongated (with high wing loading and aspect ratio), enabling relatively fast flight speeds and excellent flight efficiency, suitable for long-distance travel. Although their maneuverability is relatively poor, they possess good agility, with excellent rolling capabilities for executing turns, climbs, and dives during high-speed flight. They typically fly at high speeds in a straight line at high altitudes (around 60 - 90 m above ground), preferring open airspaces. |
| Foraging: | They generally emerge from their roosts approximately half an hour after sunset, with individuals continuing to return to the roost around midnight, peaking around one hour before sunrise. |
| Diet: | Insectivorous, capturing insects in flight. As generalist feeders, their diet is highly diverse, encompassing up to 12 insect orders and the order Araneae (spiders). They adaptively adjust their foraging strategies based on the availability and diversity of prey, preferring to capture Diptera, Lepidoptera, and Coleoptera. Dipterans, which can disperse via wind currents at high altitudes, are advantageous for these high-flying bats to prey upon. |
Diet composition of T. melanopogon in China (Guangxi Province) (Wei et al., 2008)
Diet composition of T. melanopogon in Northwest Thailand (Weterings et al., 2015)
Diet composition of T. melanopogon in India (Forest) (Srinivasulu & Srinivasulu, 2005)
Diet composition of T. melanopogon in India (Sub-urban) (Srinivasulu & Srinivasulu, 2005)
Distribution
| Local: | Further research is needed |
| Global: | T. melanopogon occurs from Sri Lanka and India through S China and SE Asia into islands of Indonesia including Borneo, Sumatra, Java, Lesser Sunda Is, Sulawesi, and many smaller islands, also in the Philippines. Subspecies to which many of these populations belong are unknown. |
| T. m. melanopogon | From Java through Lesser Sundas including Lombok, Sumbawa, Moyo, Alor Islands and Timor Island |
| T. m. bicolor | East India |
| T. m. cavaticus | West Sumatra |
| T. m. fretensis | Borneo, over scattered locations from North Sabah to Sarawak and South Martapura in South Kalimantan |
Local distribution map

Global distribution map
(Bonaccorso, 2019)
Status and Conservation
| First record: | 1993 |
| Origin: | Native |
| Local status: | Further research is needed |
| National status: | Least Concern (Red List of China Vertebrates) |
| Global status: | Least Concern (IUCN Red List) |
| Potential threat: | TBC |
Echolocation
| Parameter | Value |
|---|---|
| Call structure | QCF-FM |
| Harmonic | Multi-harmonic |
| Dominant harmonic | 2nd harmonic |
| Duration | 7.87 ± 1.74 ms |
| Inter pulse interval | 61.43 ± 50.36 ms |
| Peak frequency | 30.10 ± 3.41 kHz |
| Highest frequency | 30.14 ± 2.58 kHz |
| Lowest frequency | 22.72 ± 2.62 kHz |
|
Region: |
China (Guangxi) |
| Method: | Hand release |
| Reference: | Wei et al., 2008 |
| Parameter | Value |
|---|---|
| Call structure | Shallow FM |
| Harmonic | Multi-harmonic |
| Dominant harmonic | 2nd harmonic |
| Duration | 9.30 ± 2.40 ms |
| Inter pulse interval | - |
| Peak frequency | - |
| Highest frequency | 32.50 ± 1.70 kHz |
| Lowest frequency | 20.6 ± 0.60 kHz |
|
Region: |
Vietnam |
| Method: | Hand release |
| Reference: | Thong et al., 2022 |
| Parameter | Value |
|---|---|
| Call structure | QCF-FM |
| Harmonic | Multi-harmonic |
| Dominant harmonic | 2nd harmonic |
| Duration | 4.01 ± 0.40 ms |
| Inter pulse interval | - |
| Peak frequency | 28.40 ± 0.31 kHz |
| Highest frequency | 29.90 ± 0.21 kHz |
| Lowest frequency | 22.18 ± 0.16 kHz |
|
Region: |
Philippines |
| Method: | Flight tent |
| Reference: | Duco et al., 2023 |
Bibliography
Ades, G. (1993). New bat species for Hong Kong. Porcupine! Newsletter of the Department of Ecology & Biodiveristy, The University of Hong Kong, 8/9, 1.
Badwaik, N. (1992). Seasonal migration of two species of microchiroptera in relation to breeding cycles. Mammalia, 56(2), 287-290.
Bonaccorso, F. (2019). Emballonuridae. In Mittermeier, R. A., & Wilson, D. E. (Eds.), Handbook of the Mammals of the World – Volume 9 Bats (pp. 350-373). Lynx Edicions.
Duco, R. A., de Guia, A. P., Dimalibot, J., Alviola, P., & Gonzalez, J. C. (2023). Echolocation call characterization of insectivorous bats from caves and karst areas in southern Luzon Island, Philippines. Journal of Threatened Taxa, 15(10), 23931-23951.
Gopalakrishna, A. (1986). Migratory pattern of some Indian bats. Myotis, 23, 223-227.
Kusuminda, T. T., Edirisinghe, G. W., Nanayakkara, R. P., & Vishvanath, N. (2013). Diversity and Population status of Bats in Pilikuttuwa ancient cave temple in the Gampaha District, Sri Lanka. Asian Journal of Conservation Biology, 2(2), 136-143.
Liu, S. Y., Wu, Y., & Li, S. (2022). Handbook of the mammals of China (3rd ed.). The Straits Publishing & Distribution Group.
Muñoz‐Romo, M., Page, R. A., & Kunz, T. H. (2021). Redefining the study of sexual dimorphism in bats: following the odour trail. Mammal Review, 51(2), 155-177.
Nowak, R. M., & Paradiso, J. L. (1983). Walker’s Mammals of the World, 4th Edition. Vol. I. The Johns Hopkins University Press, 218-219.
Jeyapraba, L., Margaret, I. V., Addline, D., & Sakthi, V. (2023). Prediction of foraging strategy of insectivorous bats through their wing morphology. Journal of Survey in Fisheries Sciences, 10(3S), 1903-1917.
Rison, K., & Ngamprasertwong, T. (2016). Estimating population size of black beard tomb bats (Taphozous melanopogon) from their emergence activity. Proceeding of 11th Conference on Science and Technology for Youth (2016), 47-54.
Ruadreo, N., Voigt, C. C., & Bumrungsri, S. (2018). Large dietary niche overlap of sympatric open-space foraging bats revealed by carbon and nitrogen stable isotopes. Acta Chiropterologica, 20(2), 329-341.
Smith, A. T., Xie, Y., Hoffmann, R. S., Lunde, D., MacKinnon, J., Wilson, D. E., & Wozencraft, W. C. (Eds.). (2010). A guide to the mammals of China. Princeton University Press.
Smith, A. T., and Y. Xie (eds.). 2013. Mammals of China. Princeton University Press, Princeton, New Jersey: 246.
Srinivasulu, B., & Srinivasulu, C. (2005). Diet of the black-bearded tomb bat Taphozous melanopogon Temminck, 1841 (Chiroptera: Emballonuridae) in India. Zoos’ Print Journal, 20(8), 1935-1938.
Thong, V. D., Denzinger, A., Long, V., Sang, N. V., Huyen, N. T. T., Thien, N. H., Luong, N. K., Tuan, L. Q., Ha, N. N., Luong, N. T., & Schnitzler, H. U. (2022). Importance of mangroves for bat research and conservation: A case study from Vietnam with notes on echolocation of Myotis hasselti. Diversity, 14(4), 258.
Wei, L., Han, N., Zhang, L., Helgen, K. M., Parsons, S., Zhou, S., & Zhang, S. (2008). Wing morphology, echolocation calls, diet and emergence time of black-bearded tomb bats (Taphozous melanopogon, Emballonuridae) from southwest China. Acta Chiropterologica, 10(1), 51-59.
Wei, L., Zhou, S. Y., Zhang, L. B., Liang, B., Hong, T. Y., & Zhang, S. Y. (2006). Characteristics of echolocation calls and summer diet of three sympatric insectivorous bats species. Zoological Research, 27(3), 235.
Wei, F. W., Yang, Q. S., Wu, Y., Jiang, X. L., Liu, S. Y., Li, B. G., Yang, G., Li, M., Zhou, J., Li, S., Zhou, J., Li, S., Hu, Y. B., Ge, D. Y., Li, S., Yu, W. H., Chen, B. Y., Zhang, Z. J., Zhou, C. Q., Wu, S. B., Zhang, L., Chen Z. Z., Chen, S. D., Deng, H. Q., Jiang, T. L., Zhang, L. B., Shi, H. Y., Lu, X. L., Li, Q., Liu, Z., Cui, Y. Q., & Li, Y. C. (2021). Catalogue of mammals in China (2021). Acta Theriologica Sinica, 41(5), 487–501.
Weterings, R., Wardenaar, J., Dunn, S., & Umponstira, C. (2015). Dietary analysis of five insectivorous bat species from Kamphaeng Phet, Thailand. Raffles bulletin of zoology, 63.
Hong Kong Bat Radar. (18/05/2025). A Field Guide to Bats of Hong Kong: Black-bearded Tomb Bat (Taphozous melanopogon ). https://hkbatradar.com/en/taphozous_melanopogon